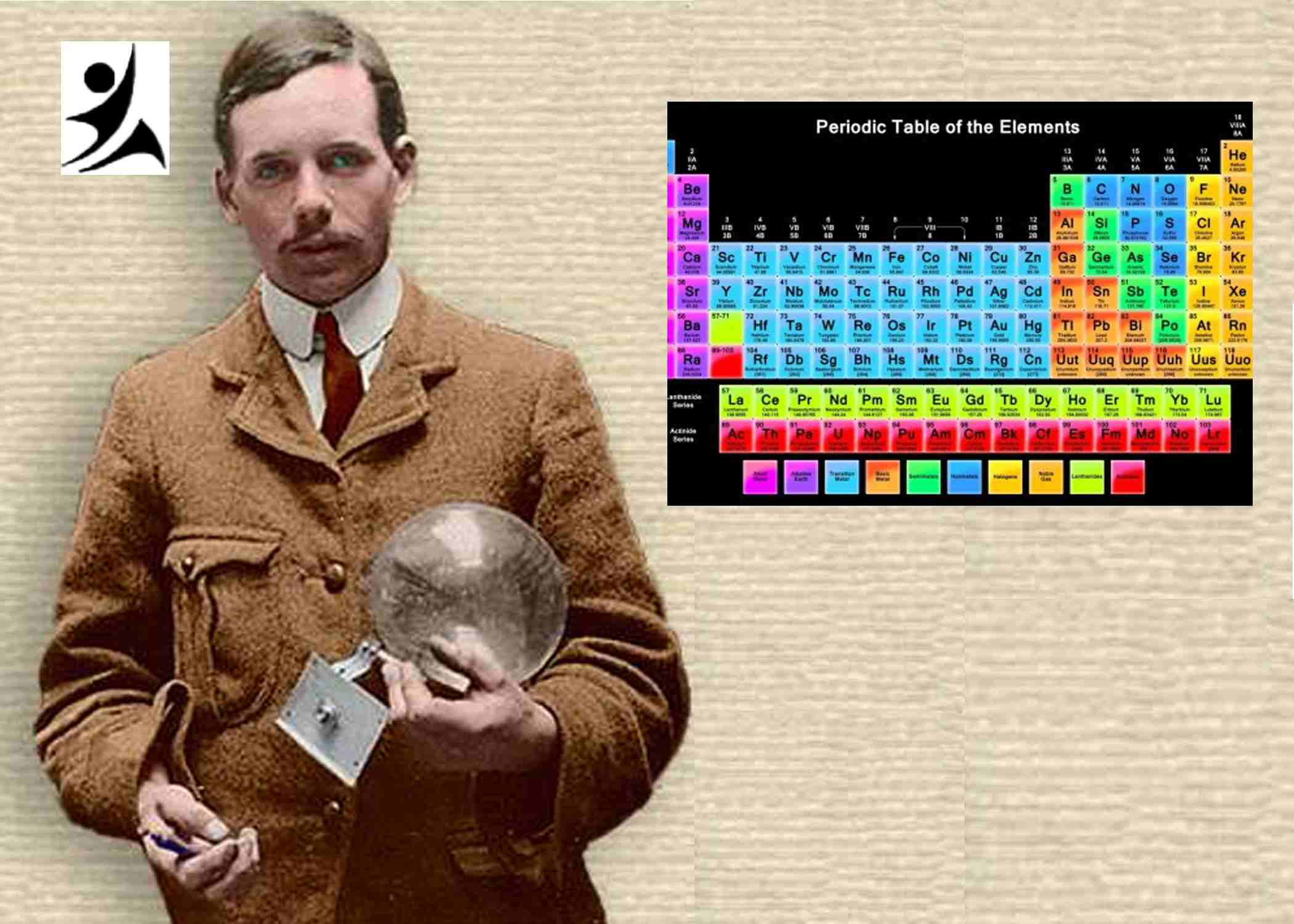
Henry Gwyn Jeffreys Moseley, a British physicist, played a pivotal role in advancing our understanding of the elements and their organization. Born on November 23, 1887, in Weymouth, Dorset, England, Moseley’s groundbreaking work on X-ray spectroscopy and the identification of atomic numbers revolutionized the Periodic Table. This extensive article delves into the life, contributions, and lasting legacy of Henry Moseley, emphasizing the names of inventors, key words, keywords, key phrases, and significant locations.
Henry Moseley attended Eton College before pursuing his undergraduate studies at Trinity College, Oxford. His scientific prowess became evident during his time at Oxford, where he focused on the study of physics. Moseley’s passion for research and experimentation would drive his future groundbreaking discoveries.
X-ray Spectroscopy and the Periodic Table
Moseley’s most significant contribution to the field of chemistry came through his work on X-ray spectroscopy. In 1913, he conducted experiments using X-rays to investigate the characteristic X-ray emissions of various elements. These experiments led him to make a groundbreaking discovery.
Atomic Numbers
Moseley observed a distinct relationship between the wavelength of the X-ray emissions and the atomic number of the elements. He proposed that the fundamental property governing the organization of elements should be the atomic number—the number of protons in an atom’s nucleus—rather than the previously used atomic mass.
By aligning the elements based on their atomic numbers, Moseley was able to rectify several inconsistencies and irregularities that existed in the previous classifications of elements. His new ordering principle aligned the elements in a way that reflected their chemical and physical properties more accurately, emphasizing the fundamental role of atomic number in determining an element’s characteristics.
Moseley’s discovery of the relationship between X-ray emissions and atomic number had profound implications for the Periodic Table and the understanding of atomic structure. His work not only provided a more accurate basis for organizing the elements but also laid the foundation for the subsequent development of quantum mechanics and the understanding of atomic structure.
Recognition and Tragic Loss
Tragically, Henry Moseley’s brilliant scientific career was cut short by his untimely death during World War I. He volunteered for military service and tragically lost his life at the age of 27 on August 10, 1915, during the Battle of Gallipoli in Turkey. Despite his premature passing, Moseley’s contributions left an indelible mark on the field of chemistry and solidified his place among the great scientific minds of his time.
Throughout his scientific journey, Henry Moseley made significant contributions to the field of physics and chemistry in various locations. Notable places associated with his work include:
- Weymouth, Dorset, England: Moseley’s birthplace in Weymouth, Dorset, served as the starting point of his scientific journey.
- Eton College: Moseley received his early education at Eton College, where he began to develop his scientific curiosity and intellectual abilities.
- Trinity College, Oxford: Moseley pursued his undergraduate studies at Trinity College, Oxford, where he furthered his knowledge of physics and conducted pioneering research.
- Turkey: Moseley tragically lost his life while serving in the military during World War I at the Battle of Gallipoli in Turkey.
Conclusion
In conclusion, Henry Gwyn Jeffreys Moseley’s contributions to the field of chemistry and physics, particularly his work on X-ray spectroscopy and the identification of atomic numbers, have had a profound and lasting impact on our understanding of the Periodic Table and atomic structure. By establishing the concept of atomic numbers as the organizing principle for elements, Moseley revolutionized the field and provided a more accurate framework for classifying and studying the elements.
Moseley’s groundbreaking experiments using X-rays not only revealed a distinct relationship between the wavelength of X-ray emissions and atomic numbers but also led to the rectification of inconsistencies and irregularities in the previous classifications of elements. His new ordering principle, based on atomic numbers rather than atomic mass, provided a more precise representation of the elements’ chemical and physical properties.
The significance of Moseley’s work extended beyond the realm of chemistry, as it laid the foundation for further advancements in atomic theory and the development of quantum mechanics. His discoveries paved the way for a deeper understanding of atomic structure and the arrangement of electrons within atoms, contributing to the fundamental principles that underpin modern physics.
Despite his tragically short life, Henry Moseley’s scientific brilliance and unwavering dedication to research left an indelible mark on the scientific community. His contributions continue to shape our understanding of the Periodic Table and have provided a solid foundation for ongoing research and discoveries in chemistry and physics.
References:
- Moseley, H.G.J. (1913). “The High-Frequency Spectra of the Elements and the Periodic Law.”
- Rutherford, E. (1911). “The Scattering of α and β Particles by Matter and the Structure of the Atom.”
- Bohr, N. (1913). “On the Constitution of Atoms and Molecules.”
- Sommerfeld, A. (1916). “On the Quantum Theory of Spectra.”