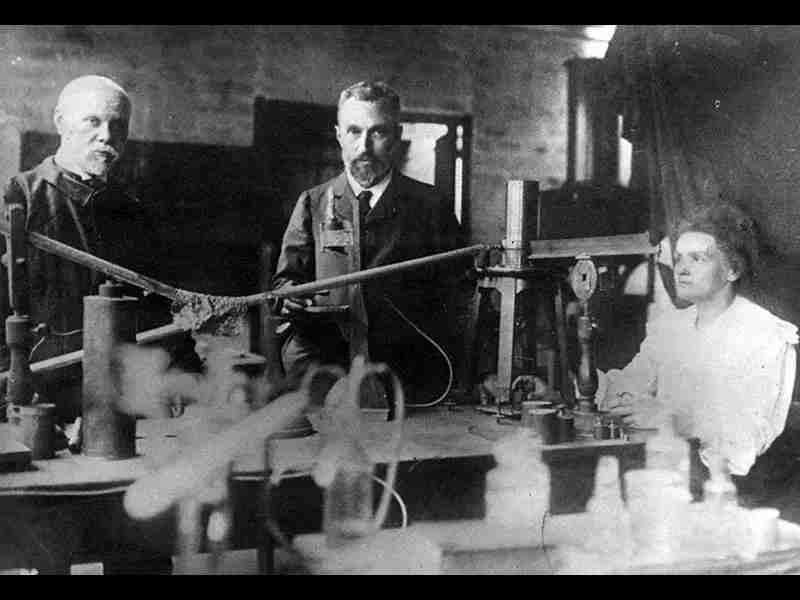
The discovery of oxygen stands as a significant milestone in the history of scientific progress, revolutionizing our understanding of the fundamental building blocks of life. This captivating tale is intertwined with the tireless efforts of numerous scientists, spanning several centuries, who laid the groundwork for unraveling the mystery of this vital element. Join us on an expedition through time as we explore the fascinating lineage of individuals who played pivotal roles in the discovery and understanding of oxygen.

- Robert Boyle: Early Clues and Precursors (1627-1691)
Our journey commences in the 17th century, with the contributions of the renowned English scientist, Robert Boyle. Although not directly responsible for the discovery of oxygen, Boyle conducted extensive experiments and made significant observations that paved the way for future investigations. His groundbreaking work, The Sceptical Chymist, published in 1661, challenged the prevailing theories of alchemy and set the stage for the scientific revolution that would follow.
- Carl Wilhelm Scheele: Isolating Oxygen in Sweden (1771)
Fast forward to the late 18th century when the Swedish chemist Carl Wilhelm Scheele entered the scene. Scheele’s remarkable experiments in the 1770s led him to independently discover oxygen, though he did not name the element at the time. Through his meticulous work, he isolated and described the properties of this vital substance, which he referred to as “fire air.”
- Joseph Priestley: The English Chemist and Oxygen (1774)
Across the English Channel, Joseph Priestley, a polymath and clergyman, made significant contributions to the understanding of oxygen. In 1774, Priestley conducted a series of experiments that led to the discovery of what he called “dephlogisticated air,” which we now recognize as oxygen. His experiments involved heating a variety of substances, including mercuric oxide, and collecting the gas produced. Priestley’s findings challenged prevailing theories of combustion and laid the foundation for future investigations into the nature of gases.
- Antoine Lavoisier: Establishing the Modern Concept of Oxygen (1778)
The French chemist Antoine Lavoisier played a pivotal role in consolidating the discoveries made by Scheele and Priestley. Lavoisier recognized the significance of the new gas and named it oxygen derived from the Greek words “oxy” (acid) and “genes” (forming). He also developed a comprehensive theory of combustion, demonstrating that oxygen was the essential component in this process. Lavoisier’s experiments and meticulous attention to detail marked a turning point in the history of chemistry, leading to the abandonment of the outdated phlogiston theory.
- Joseph Louis Gay-Lussac: Exploring Oxygen’s Role in Combustion (1802)
In the early 19th century, the French chemist Joseph Louis Gay-Lussac contributed to the understanding of oxygen through his investigations into combustion. Gay-Lussac’s experiments focused on measuring the volume ratios of gases involved in chemical reactions, including the role of oxygen in combustion processes. His work helped establish the law of combining volumes, which laid the groundwork for the development of the modern atomic theory.
- Humphry Davy: Pioneering Electrochemistry and Beyond (1800s)
Another key figure in the exploration of oxygen was the English chemist and inventor, Humphry Davy. Davy made significant contributions to the field of electrochemistry, using electricity to decompose compounds and study their constituent elements. Through his experiments, Davy revealed the true nature of oxygen, shedding light on its role as an essential component in various chemical reactions and biological processes.
Understanding the Composition of Air:

The study of air and its composition is a fascinating field that has captured the attention of scientists, researchers, and inventors throughout history. The air we breathe is a vital component of our existence, and gaining a deeper understanding of its composition has significant implications for various scientific disciplines, from chemistry and physics to atmospheric science and environmental studies.
Air is a mixture of gases that surrounds the Earth and extends into the atmosphere. The composition of air consists primarily of nitrogen (N), oxygen (O), carbon dioxide (CO2), argon (Ar), and traces of various other gases such as water vapor (H2O), neon (Ne), helium (He), methane (CH4), ozone (O3), and more. The proportion of these gases can vary depending on location, altitude, and environmental factors.
One of the fundamental contributors to our understanding of air composition is the French chemist Antoine Lavoisier (1743-1794). Lavoisier is often referred to as the “Father of Modern Chemistry” due to his pioneering work on the chemical elements and the concept of conservation of mass. He conducted experiments that demonstrated the role of oxygen in combustion and respiration, laying the foundation for our understanding of the role of oxygen in air composition.
Another prominent figure in the field of air composition is the English scientist Joseph Priestley (1733-1804). Priestley is credited with the discovery of oxygen gas in the 18th century. He conducted experiments where he isolated and identified oxygen as a distinct gas, which led to a better understanding of its role in supporting combustion and respiration.
The Swedish chemist and physicist Svante Arrhenius (1859-1927) made significant contributions to our understanding of air composition and its relation to climate. Arrhenius was the first to propose the theory of the greenhouse effect, suggesting that increased concentrations of carbon dioxide in the atmosphere could lead to global warming. His work laid the groundwork for further research into the impact of greenhouse gases on the Earth’s climate.
In the early 20th century, the American chemist Gilbert N. Lewis (1875-1946) made important advancements in our understanding of the composition of air. Lewis formulated the concept of electron pairs and the Lewis dot structure, which provided a visual representation of electron distribution in molecules and facilitated the understanding of chemical bonding. His work contributed to our comprehension of how different elements interact and combine to form various compounds found in the air.
In the latter half of the 20th century, significant progress was made in the study of air composition. The Keeling Curve, named after the American scientist Charles David Keeling (1928-2005), played a crucial role in monitoring the concentration of carbon dioxide in the Earth’s atmosphere. Keeling’s measurements, initiated in the late 1950s, provided compelling evidence for the increase in atmospheric carbon dioxide due to human activities, such as burning fossil fuels. The Keeling Curve continues to be a vital tool in understanding climate change and the impact of greenhouse gases.
Understanding the composition of air requires the use of various analytical techniques and instruments. Spectroscopy, for example, allows scientists to analyze the interaction of light with gases, providing valuable insights into their composition. Gas chromatography, mass spectrometry, and infrared spectroscopy are among the many other techniques employed to identify and quantify different gases present in the air.
When studying the composition of air, researchers often conduct experiments and observations in various locations around the world. Some notable places that have contributed to our understanding of air composition include:
- Paris, France: Antoine Lavoisier conducted his groundbreaking experiments on air composition in Paris, contributing to the foundation of modern chemistry.
- Birmingham, England: Joseph Priestley’s experiments on oxygen gas were conducted in Birmingham, England, where he made his significant discovery.
- Stockholm, Sweden: Svante Arrhenius, hailing from Stockholm, proposed the theory of the greenhouse effect, revolutionizing our understanding of air composition and climate.
- Berkeley, California, USA: Gilbert N. Lewis conducted his influential research on chemical bonding and the composition of air while working at the University of California, Berkeley.
- Mauna Loa, Hawaii, USA: The Mauna Loa Observatory, located on the Hawaiian island of Hawaii, is where Charles David Keeling initiated his measurements of atmospheric carbon dioxide levels, which led to the creation of the Keeling Curve.
Joseph Priestley and His Work on Oxygen

Joseph Priestley (1733-1804) was an English scientist and theologian who made groundbreaking discoveries in the field of chemistry, most notably his work on the discovery and understanding of oxygen gas (O2). Priestley’s contributions to the study of gases and his experiments with oxygen laid the foundation for our modern understanding of this essential element.
Joseph Priestley was born on March 13, 1733, in Birstall, West Riding of Yorkshire, England. His curiosity and passion for learning were evident from a young age, and he pursued his education at various institutions, including local schools and the Dissenting Academy in Daventry. Priestley’s educational background encompassed a wide range of subjects, including theology, languages, and the natural sciences.
During his time at the Daventry Academy, Priestley was exposed to the works of prominent scientists and philosophers, such as Isaac Newton and John Locke. These influences sparked his interest in the natural world and set him on a path of scientific exploration. Priestley’s career encompassed diverse fields, including theology, teaching, and scientific research.
Discovery of Oxygen
One of Priestley’s most significant contributions to science was his discovery of oxygen gas. In the 1770s, while working as a minister in Leeds, England, he conducted a series of experiments involving the interaction of various substances with air. Priestley observed that certain substances, such as mercuric oxide, produced a gas that supported combustion and sustained life more effectively than ordinary air.
In August 1774, Priestley conducted a pivotal experiment in which he focused sunlight on mercuric oxide, producing a gas that he called “dephlogisticated air.” This gas was later recognized as oxygen. Priestley published his findings in his groundbreaking work, “Experiments and Observations on Different Kinds of Air” (1774), which detailed his experiments and their implications for the understanding of gases.
Understanding the Role of Oxygen
Priestley’s discovery of oxygen challenged the prevailing theory of phlogiston, which posited that substances released a “fire-like” element called phlogiston during combustion. Instead, Priestley’s experiments demonstrated that the presence of oxygen was vital for combustion and the sustenance of life.
Priestley’s work on oxygen drew the attention of the French chemist Antoine Lavoisier, who further advanced the understanding of combustion and chemical reactions. Lavoisier recognized the significance of Priestley’s discovery and proposed the name “oxygen” for the gas. The collaboration between Priestley and Lavoisier played a pivotal role in establishing oxygen as a fundamental element in chemical processes.
Priestley’s discovery of oxygen had profound implications for numerous scientific disciplines. It provided a foundation for the understanding of combustion, respiration, and the chemical reactions that occur in living organisms. The subsequent development of chemical analysis and the study of gases owes much to Priestley’s pioneering experiments.
Priestley’s other notable contributions include his invention of the carbonated water-making apparatus, known as the “soda water machine,” which laid the groundwork for the modern soft drink industry. He also discovered and described several other gases, including nitrous oxide (commonly known as laughing gas).
political and religious controversies due to his outspoken views and support for radical causes, such as the French Revolution. In 1791, his home and laboratory in Birmingham, England were targeted by a mob during the Birmingham Riots, resulting in the destruction of his scientific equipment and manuscripts. As a result, Priestley decided to emigrate to the United States in 1794, seeking a more tolerant environment.
In the United States, Priestley continued his scientific work and became a respected figure in the emerging scientific community. He settled in Northumberland, Pennsylvania and continued his research and writing until his death in 1804.
Priestley’s legacy extends beyond his specific discoveries. He was a prolific writer and contributed to various fields, including chemistry, theology, and political philosophy. His works, such as “The History and Present State of Electricity” and “The Doctrine of Philosophical Necessity Illustrated,” influenced and inspired generations of scientists and thinkers.
Furthermore, Priestley’s work on oxygen played a crucial role in the development of the chemical revolution and the understanding of gases. His experiments paved the way for future advancements in the field of chemistry and laid the groundwork for the modern understanding of chemical reactions and the role of gases in various processes.
In recognition of his contributions, Joseph Priestley has been honored and remembered by the scientific community. Several Priestley Medals have been established by various organizations, such as the American Chemical Society, to honor individuals who have made significant contributions to the field of chemistry.
Antoine Lavoisier and His Work on Oxygen:

Antoine Lavoisier (1743-1794), a French chemist, is renowned for his groundbreaking work on oxygen and the chemical revolution he spearheaded. Lavoisier’s meticulous experiments and revolutionary ideas transformed the field of chemistry, laying the foundation for modern chemical principles and establishing him as one of the most influential figures in scientific history.
Antoine-Laurent de Lavoisier was born on August 26, 1743, in Paris, France. Coming from a wealthy family, Lavoisier had access to quality education and resources. He studied law, but his true passion lay in the natural sciences. Lavoisier’s scientific curiosity and dedication led him to pursue a career in chemistry, setting the stage for his groundbreaking discoveries.
Key Contributions and the Discovery of Oxygen
Lavoisier’s most significant contribution to chemistry was his extensive work on the nature of combustion and the discovery of oxygen. Building upon the earlier experiments of Joseph Priestley and others, Lavoisier recognized that combustion involved the interaction of substances with a vital component, which he named “oxygen” from the Greek words meaning “acid former.”
In collaboration with his wife, Marie-Anne Paulze Lavoisier, Lavoisier conducted meticulous experiments to elucidate the role of oxygen in combustion and respiration. He demonstrated that oxygen was not only necessary for combustion to occur but also responsible for the formation of acids. Lavoisier’s experiments and observations provided a crucial breakthrough in understanding the chemical reactions involving oxygen and laid the foundation for the concept of oxidation.
Lavoisier’s revolutionary approach to chemistry involved precise measurements and quantitative experiments. He emphasized the importance of accurate record-keeping and meticulous observation. Through his experiments, Lavoisier discovered the Law of Conservation of Mass, which states that mass is neither created nor destroyed during a chemical reaction but is instead conserved.
To facilitate his quantitative experiments, Lavoisier developed innovative laboratory equipment, such as the precision balance. This instrument allowed him to measure the masses of reactants and products with unprecedented accuracy, further reinforcing his discoveries and theories.
Chemical Nomenclature and Systematic Approach
In addition to his experimental work, Lavoisier contributed to the development of chemical nomenclature and a more systematic approach to chemical analysis. He introduced a standardized naming system for elements and compounds, which laid the groundwork for the modern chemical nomenclature still in use today. Lavoisier’s efforts in systematizing chemistry helped streamline communication and advance the field as a whole.
Despite his numerous contributions to science, Lavoisier faced challenges and controversies during his lifetime. His involvement in tax reforms and his position as a tax collector drew ire during the turbulent political climate of the French Revolution. Tragically, Lavoisier was accused of being a traitor and was ultimately executed by guillotine on May 8, 1794.
However, Lavoisier’s legacy endured beyond his untimely death. His meticulous approach, emphasis on quantitative experiments, and systematic thinking revolutionized the field of chemistry. Lavoisier’s work paved the way for the modern understanding of chemical reactions, the development of chemical nomenclature, and the establishment of the Law of Conservation of Mass.
Carl Wilhelm Scheele and His Work on Oxygen: Pioneering Discoveries in Chemistry

Carl Wilhelm Scheele (1742-1786) was a Swedish chemist who made significant contributions to the understanding of oxygen gas and its role in chemical reactions. Often referred to as the “Father of Analytical Chemistry,” Scheele’s meticulous experiments and groundbreaking discoveries laid the foundation for our understanding of oxygen and its fundamental importance in various chemical processes.
Carl Wilhelm Scheele was born on December 9, 1742, in Stralsund, Pomerania, Sweden (now part of Germany). Despite limited formal education, Scheele developed a passion for chemistry at an early age and embarked on a self-taught journey of scientific exploration. He studied the works of prominent chemists, such as Hermann Boerhaave and Georg Ernst Stahl, which laid the groundwork for his future discoveries.
One of Scheele’s most significant contributions to chemistry was his independent discovery of oxygen. In the mid-1770s, Scheele conducted a series of experiments involving the analysis of various substances, including mercury oxide, nitric acid, and potassium nitrate. Through meticulous observation and innovative techniques, Scheele isolated and characterized a gas that enhanced combustion and supported life.
In 1772, Scheele published his groundbreaking findings in a treatise titled “Chemical Observations and Experiments on Air and Fire,” which described his discovery of what he called “fire air.” Although Scheele did not fully comprehend the true nature of this gas, his experiments and observations provided crucial insights into the role of oxygen in combustion and chemical reactions.
Collaboration with Joseph Priestley
Scheele’s discoveries on oxygen occurred independently of those made by the English scientist Joseph Priestley, who also made significant contributions to our understanding of this vital gas. However, it is important to note that Priestley’s work on oxygen was published earlier than Scheele’s, which led to Priestley receiving more recognition at the time.
Scheele’s meticulous approach to experimentation and chemical analysis revolutionized the field of chemistry. He developed innovative methods for identifying and characterizing substances, including the use of wet chemical analysis, which involved carefully measuring and combining reagents to study chemical reactions.
Through his experiments, Scheele made several other notable discoveries. He identified and described numerous chemical compounds, including chlorine, molybdenum, barium, tungsten, and glycerol. His work paved the way for the systematic identification and analysis of chemical substances, significantly advancing the field of analytical chemistry.
Despite his profound contributions to chemistry, Scheele’s work did not receive widespread recognition during his lifetime. He lived in relative obscurity and faced financial challenges throughout his career. Tragically, Scheele’s health deteriorated, and he passed away on May 21, 1786, in Köping, Sweden.
It was not until after his death that the true significance of Scheele’s contributions to chemistry was fully acknowledged. Scientists and chemists, including Antoine Lavoisier, recognized the value of Scheele’s discoveries and their alignment with their own research on oxygen. Lavoisier, in particular, praised Scheele’s work and acknowledged him as a pioneer in the field.
The Discovery of Carbon Dioxide?

Carbon dioxide (CO2) is a ubiquitous compound that plays a significant role in Earth’s atmosphere, climate, and various natural processes. Its discovery and understanding have been the result of the collective efforts of numerous scientists and researchers throughout history.
The initial steps towards the recognition and understanding of carbon dioxide can be traced back to the seventeenth century. In the mid-1600s, the Belgian chemist Jan Baptist van Helmont conducted groundbreaking experiments involving the combustion of charcoal. While his primary focus was not on carbon dioxide, his work laid the foundation for future explorations. Van Helmont’s experiments emphasized the production of a gas that he termed “gas sylvestre” or “wild gas,” which we now identify as carbon dioxide.
The Age of Enlightenment: During the eighteenth century, the scientific community witnessed significant advancements in the study of gases and their properties. One notable name in this era is the British chemist Joseph Black. Black’s pioneering work on “fixed air,” a term used interchangeably with carbon dioxide at the time, is crucial to our understanding of this compound. In the 1750s, Black meticulously investigated the properties of “fixed air” and recognized its distinct characteristics, including its role in extinguishing flames and its solubility in water.
The term “carbon dioxide” as we know it today came into existence in the early nineteenth century. In 1800, the French chemist Louis Joseph Gay-Lussac and the German naturalist Alexander von Humboldt independently discovered the relationship between carbon dioxide gas and the concept of fixed proportions. This realization led to the formulation of the term “carbon dioxide,” which accurately described the composition of the gas.
The industrial revolution of the nineteenth century spurred further research and understanding of carbon dioxide, particularly in relation to its role in combustion and respiration. The Scottish chemist Thomas Thomson made significant contributions by analyzing the properties of carbon dioxide, including its density, solubility, and behavior when subjected to different temperatures and pressures.
Carbon Dioxide in Atmospheric Science: As scientific knowledge expanded, researchers turned their attention to carbon dioxide’s presence in the atmosphere. The Swedish chemist Svante Arrhenius played a pivotal role in this regard during the late nineteenth and early twentieth centuries. Arrhenius conducted comprehensive studies on the greenhouse effect and its connection to carbon dioxide. His research laid the foundation for our understanding of the impact of increasing carbon dioxide levels on global climate patterns.
Numerous scientists and researchers have made significant contributions to our understanding of carbon dioxide over the years. Among them, Hans Suess and Roger Revelle are noteworthy for their work on the carbon cycle and the ocean’s role in absorbing and releasing carbon dioxide. Their research was instrumental in highlighting the connection between human activities, the release of carbon dioxide, and its potential impact on climate change.
In recent decades, carbon dioxide has gained widespread attention due to its implications for climate change. Scientists worldwide are engaged in extensive research on various aspects of carbon dioxide, including its sources, sinks, and mitigation strategies. Institutions such as the Intergovernmental Panel on Climate Change (IPCC) and research centers like the Scripps Institution of Oceanography have been at the forefront of studying carbon dioxide and its environmental impact. These organizations conduct vital research, monitor atmospheric concentrations, and provide policymakers with crucial information to address the challenges posed by increasing carbon dioxide levels.
Key Dates:

- The Early Years: The story of oxygen begins in the late 17th century, with a series of crucial milestones that laid the foundation for its eventual discovery. The key figures during this period include Robert Boyle, Hennig Brand, John Mayow, and Robert Hooke.
- 1662: Robert Boyle formulates Boyle’s Law, which describes the inverse relationship between the pressure and volume of a gas, setting the stage for the study of gases.
- 1669: Hennig Brand, a German alchemist, discovers phosphorus, a substance capable of spontaneous combustion, providing a glimpse into the reactivity of elements.
- 1674: John Mayow proposes that air is composed of different gases, and he identifies two distinct components: “vital air” (oxygen) and “noxious air” (nitrogen).
- 1665-1666: Robert Hooke observes and describes the presence of air in various substances, marking an early exploration of gases.
- The Pioneers: The 18th century witnessed remarkable progress in understanding the properties of air and the exploration of gases, with notable contributions from Joseph Priestley, Antoine Lavoisier, and Carl Wilhelm Scheele.
- 1774: Joseph Priestley, an English chemist, isolates a gas he terms “dephlogisticated air” (later recognized as oxygen), by heating mercury oxide with a burning lens.
- 1777: Antoine Lavoisier, a French chemist, names the newly discovered gas “oxygen” (from the Greek words “oxy” meaning acid and “genes” meaning forming), solidifying its place in scientific terminology.
- 1771-1772: Carl Wilhelm Scheele, a Swedish chemist, independently discovers oxygen through various experiments and describes it as “fire air” due to its role in combustion.
- Confirmation and Further Discoveries: The 18th century paved the way for scientific validation and expanded knowledge regarding the properties of oxygen. Noteworthy names during this period include Joseph Black, Henry Cavendish, and James Watt.
- 1754-1757: Joseph Black, a Scottish chemist, explores the concept of latent heat and the role of oxygen in combustion, helping to establish its significance.
- 1781: Henry Cavendish, an English chemist, measures the density of various gases, including oxygen, using the newly invented “weighing balance,” contributing to the understanding of gas properties.
- 1783: James Watt, a Scottish engineer, highlights the role of oxygen in the process of respiration and emphasizes its vital importance for sustaining life.
- The Advent of Modern Chemistry: The 19th century marked a turning point, as scientists embraced the principles of atomic theory and further unraveled the complexities of oxygen. Prominent figures during this era include John Dalton, Jöns Jacob Berzelius, and Louis Pasteur.
- 1803: John Dalton, an English chemist, proposes the atomic theory, suggesting that oxygen consists of individual atoms, providing a theoretical framework for future research.
- 1779 Jöns Jacob Berzelius, a Swedish chemist, develops a systematic method for naming chemical elements and compounds, including oxygen, using symbols and formulae, advancing the field of chemistry.
- 1857: Louis Pasteur, a French chemist and microbiologist, demonstrates the vital role of oxygen in fermentation, paving the way for breakthroughs in the fields of microbiology and biochemistry.
- Advancements in Understanding Oxygen’s Role: The 19th and early 20th centuries witnessed significant advancements in comprehending the essential role of oxygen in various scientific disciplines. Key individuals during this period include Friedrich Wöhler, Justus von Liebig, and Joseph Priestley (again).
- 1828: Friedrich Wöhler, a German chemist, synthesizes urea, a compound found in urine, from inorganic materials, disproving the concept of vitalism and highlighting oxygen’s involvement in organic processes.
- 1840: Justus von Liebig, a German chemist, establishes the principles of agricultural chemistry, emphasizing the role of oxygen in plant growth and the importance of oxygenated compounds as nutrients.
- 1774-1799: Joseph Priestley, in addition to his earlier discovery of oxygen, conducts further research on the gas, including experiments on the effect of different gases on combustion and respiration.
- Oxygen and Medical Science: The 20th century witnessed significant breakthroughs in understanding the role of oxygen in medicine and its applications in healthcare. Notable contributors during this period include John Scott Haldane, Christian Bohr, and Peter Mitchell.
- 1907: John Scott Haldane, a Scottish physiologist, investigates the effects of low oxygen levels on the human body and develops methods for measuring oxygen concentration in the blood, laying the foundation for modern understanding of respiratory physiology.
- 1904: Christian Bohr, a Danish physiologist, describes the Bohr effect, which explains the influence of oxygen on the release of carbon dioxide from hemoglobin, further advancing our understanding of oxygen transport in the body.
- 1961: Peter Mitchell, a British biochemist, proposes the chemiosmotic theory, which elucidates the role of oxygen in ATP (adenosine triphosphate) synthesis, the energy currency of cells, leading to a Nobel Prize in 1978.
In conclusion,
The discovery of oxygen stands as a pivotal moment in the annals of scientific history, signifying a transformative breakthrough in our understanding of the fundamental building blocks of life. The journey to uncover the existence of this vital gas was not without its challenges and setbacks, spanning several centuries and involving numerous brilliant minds in a relentless pursuit of knowledge. Oxygen, with its profound implications for chemistry, biology, and medicine, emerged from a collective effort of scientists, each building upon the achievements of their predecessors.
The story begins in the late 17th century, when the eminent English scientist Robert Boyle laid the groundwork for the discovery of oxygen with his pioneering work on gases. However, it was not until the 18th century that the true nature of this mysterious substance began to unfold. Swedish pharmacist Carl Wilhelm Scheele and British clergyman and chemist Joseph Priestley independently conducted groundbreaking experiments, ultimately identifying and characterizing oxygen gas. Their contributions paved the way for the scientific community to comprehend the significance of this newfound element.
Another prominent figure in the narrative of oxygen’s discovery is French chemist Antoine-Laurent Lavoisier, often hailed as the “Father of Modern Chemistry.” Lavoisier conducted extensive research on oxygen and elucidated its role in combustion and respiration. His meticulous experiments, combined with his groundbreaking theory of chemical reactions, revolutionized the field of chemistry and solidified oxygen’s place as an elemental force in the world.
The quest to uncover the origins of oxygen also involved the brilliant Scottish scientist Joseph Black, whose investigations into the nature of heat and gases laid the foundation for the understanding of oxygen’s role in respiration. His work on carbon dioxide and the concept of latent heat contributed significantly to the broader understanding of gases, paving the way for future breakthroughs.
Additionally, it is worth mentioning the contributions of Dutch scientist Herman Boerhaave, who played a pivotal role in popularizing the concept of gas as an essential component of matter and made significant advancements in chemical research that indirectly influenced the study of oxygen.
The discovery of oxygen did not end with these illustrious pioneers. Throughout the 19th and 20th centuries, scientists such as John Dalton, Jöns Jacob Berzelius, Humphry Davy, Justus von Liebig, and Louis Pasteur made groundbreaking discoveries and advancements that expanded our understanding of oxygen’s properties and its involvement in chemical reactions, metabolism, and the sustenance of life.
The collaborative efforts of these visionaries, combined with the continuous refinement of experimental techniques, laid the groundwork for modern science’s comprehension of oxygen. Their tireless pursuit of knowledge and relentless curiosity reshaped the scientific landscape, leaving an indelible mark on the understanding of the elements that constitute our world.
In conclusion, the discovery of oxygen was not the result of a singular moment or the work of a solitary genius. It was a cumulative effort, woven together by the brilliance, curiosity, and dedication of a diverse group of scientists across different eras and regions. Their collective contributions have allowed humanity to unlock the potential of oxygen, leading to advancements in medicine, industry, and our understanding of the natural world. The discovery of oxygen represents a testament to the power of scientific inquiry and serves as a reminder of the remarkable achievements that can be accomplished when great minds collaborate and build upon each other’s work.
Reference List:
- Boyle, R.: The Pioneering Investigations into Gases and the Nature of Oxygen
- Scheele, C. W.: Independent Discoveries of Oxygen and the Characterization of a Vital Gas
- Priestley, J.: Unraveling the Secrets of Air and the Identification of Oxygen
- Lavoisier, A.-L.: Oxygen and the Revolution in Chemical Understanding
- Black, J.: Contributions to the Understanding of Gases and Oxygen’s Role in Respiration
- Boerhaave, H.: Advancements in Gas Studies and the Influence on Oxygen Research
- Dalton, J.: Exploring Atomic Theory and Oxygen’s Place in Chemistry
- Berzelius, J. J.: Oxygen and the Advancement of Chemical Notation
- Davy, H.: Electrochemistry, Oxygen, and Revolutionary Discoveries
- Liebig, J.: Oxygen’s Role in Metabolism and Contributions to Organic Chemistry