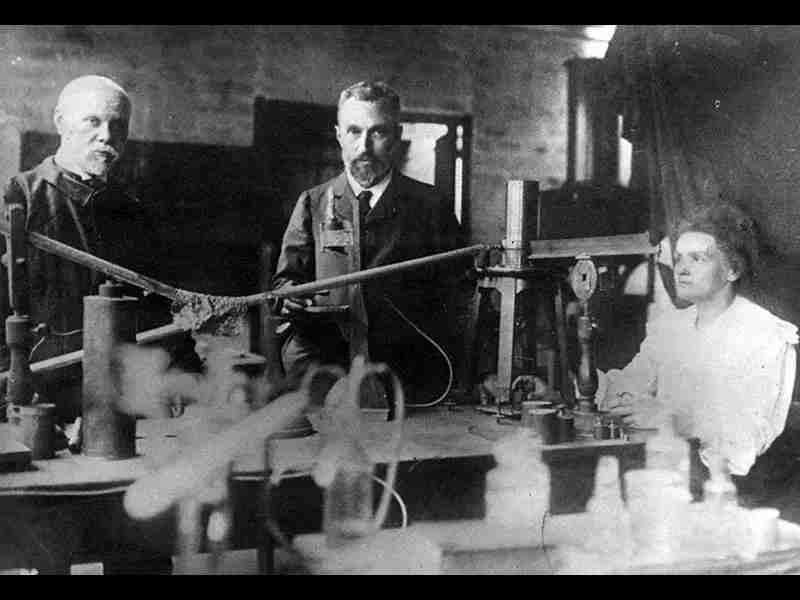
The story of the discovery of radioactivity is a fascinating journey that led to a revolution in our understanding of the fundamental nature of matter. This groundbreaking discovery, which unveiled a previously unknown realm of atomic processes, was the culmination of the efforts of several brilliant scientists and researchers. From the early experiments to the identification of key radioactive elements, this article aims to explore the names, places, and pivotal moments that shaped the discovery of radioactivity.

Henri Becquerel and Wilhelm Roentgen The history of radioactivity began in the late 19th century when Henri Becquerel, a French physicist, made a serendipitous discovery. In 1896, Becquerel was conducting experiments with uranium salts and photographic plates when he noticed that the plates were being exposed, even though they were wrapped in opaque material. This led him to conclude that uranium emitted a type of radiation capable of penetrating solid objects. Becquerel’s accidental discovery laid the foundation for the subsequent exploration of radioactivity.
Around the same time, another influential scientist, Wilhelm Roentgen, a German physicist, made a breakthrough discovery related to X-rays. In 1895, while studying cathode rays, Roentgen noticed that a fluorescent screen in his lab began to emit a glow, even though it was shielded from the rays. Further investigations revealed the existence of X-rays, a form of high-energy electromagnetic radiation. Although not directly related to radioactivity, Roentgen’s work was instrumental in the broader field of radiation research.
Marie Curie, a Polish-born physicist, and her husband Pierre Curie played an instrumental role in isolating and studying radioactive elements. In 1898, the Curies announced their discovery of a new radioactive element, polonium, named after Marie Curie’s native Poland. Shortly thereafter, they discovered another radioactive element, which they named radium. Their work involved painstakingly extracting these elements from pitchblende, a mineral ore that contains uranium.
The Curies’ groundbreaking research, conducted in their laboratory in Paris, not only led to the identification of new elements but also provided a deeper understanding of the properties of radioactivity. Marie Curie’s tireless efforts, including her development of techniques for isolating radium in significant quantities, earned her two Nobel Prizes—one in Physics (1903) and another in Chemistry (1911). The Curies’ work laid the foundation for further advancements in the field of radioactivity.
Another key figure in the discovery of radioactivity was Ernest Rutherford, a New Zealand-born physicist. Rutherford conducted a series of experiments in the early 20th century that led to a new understanding of the structure of the atom. Through his famous gold foil experiment, conducted in 1911 at the University of Manchester, Rutherford demonstrated that the atom has a dense, positively charged nucleus at its center, with electrons orbiting around it.
Rutherford’s work not only confirmed the existence of radioactivity but also paved the way for the development of nuclear physics. His research on radioactive decay and the transmutation of elements provided crucial insights into the atomic nucleus and led to the discovery of isotopes.
The Mystery of Radioactivity: A Comprehensive Exploration

Radioactivity, a fascinating phenomenon that revolutionized our understanding of matter, energy, and the building blocks of the universe, has captivated scientists and researchers for over a century. Becquerel and Roentgen The journey to unraveling the secrets of radioactivity began with the remarkable discoveries of Henri Becquerel and Wilhelm Roentgen. In 1896, Becquerel, a French physicist, accidentally stumbled upon radioactivity while studying uranium salts. His experiments revealed that certain materials emitted radiation, a term used to describe the emission of energy in the form of particles or electromagnetic waves. This accidental discovery laid the foundation for the subsequent exploration of radioactivity.
Around the same time, Roentgen, a German physicist, made a groundbreaking discovery of his own. In 1895, while investigating cathode rays, he stumbled upon a new type of penetrating radiation, which he termed X-rays. Roentgen’s discovery opened up new avenues of exploration in the field of radiation and laid the groundwork for further discoveries related to radioactivity.
The Curies and Rutherford One of the most prominent names associated with radioactivity is that of Marie Curie, a Polish-born physicist, and her husband, Pierre Curie. In the late 19th century, the Curies conducted groundbreaking research on radioactive elements. They discovered and isolated two new elements: polonium and radium. Marie Curie’s meticulous work in isolating these elements from pitchblende, a mineral ore containing uranium, not only expanded our understanding of radioactivity but also earned her two Nobel Prizes—one in Physics (1903) and another in Chemistry (1911).
Another significant contributor to our understanding of radioactivity was Ernest Rutherford, a New Zealand-born physicist. Rutherford’s experiments, conducted at the University of Manchester, led to groundbreaking insights into the structure of the atom and the nature of radioactivity. His famous gold foil experiment in 1911 demonstrated that atoms have a dense, positively charged nucleus at their center, with negatively charged electrons orbiting around it. This discovery paved the way for further advancements in nuclear physics and the understanding of radioactive decay.
Concepts and Significance Central to the understanding of radioactivity is the concept of radioactive decay. Radioactive isotopes, such as uranium or radium, undergo spontaneous decay, emitting particles or energy in the process. This decay follows a predictable pattern, and the rate at which it occurs is measured by the half-life—the time required for half of a radioactive substance to decay into a stable form. The half-life allows scientists to determine the longevity and potential hazards associated with radioactive materials.
Applications and Dangers of Radioactivity The discovery of radioactivity has had profound implications in various fields. In medicine, radiotherapy harnesses the power of radiation to treat cancerous tumors. Diagnostic tools like X-ray imaging and radiation therapy have transformed medical practices and saved countless lives. Additionally, radioisotopes find applications in industry, archaeology, and environmental studies.
However, it is crucial to acknowledge the potential dangers associated with radioactivity. Exposure to high levels of radiation can have severe health effects, including radiation sickness, genetic mutations, and an increased risk of developing cancer. Protective measures and strict regulations are necessary to ensure the safe handling and disposal of radioactive materials.
Notable Places and Contributions: Throughout the history of radioactivity, certain places have emerged as epicenters of scientific breakthroughs and discoveries. Paris, France, played a pivotal role in the study of radioactivity, particularly with the groundbreaking work conducted by Marie and Pierre Curie at the University of Paris. Their laboratory became a hub for radiation research and attracted scientists from around the world.
The University of Manchester, in the United Kingdom, gained prominence due to the research conducted by Ernest Rutherford. His experiments on radioactivity and the structure of the atom revolutionized our understanding of nuclear physics and paved the way for future advancements in the field.
The Manhattan Project & the Cold War

The Manhattan Project, named after the borough of Manhattan in New York City, was a top-secret research and development endeavor initiated during World War II. Its primary objective was to harness the power of nuclear energy and create an atomic bomb. The project brought together a remarkable group of scientists, including notable names such as J. Robert Oppenheimer, Enrico Fermi, and Leo Szilard.
J. Robert Oppenheimer, an American theoretical physicist, played a key role as the scientific director of the Manhattan Project. His leadership and intellect were instrumental in bringing together the diverse talents necessary to develop the atomic bomb. Enrico Fermi, an Italian physicist, made significant contributions to the project, including overseeing the construction and operation of the first nuclear reactor at the University of Chicago.
Los Alamos, a remote location in New Mexico, became the nerve center for the Manhattan Project. It was here that the brilliant minds collaborated and conducted groundbreaking research to unlock the secrets of nuclear fission. Led by Oppenheimer, scientists worked tirelessly at the Los Alamos National Laboratory to design and assemble the first atomic bomb.
The project reached its pinnacle on July 16, 1945, with the successful test of the world’s first atomic bomb, code-named Trinity, in the New Mexico desert. This pivotal moment marked the dawn of a new era, where mankind had harnessed the immense power of the atom.
The completion of the atomic bomb coincided with the closing stages of World War II. On August 6, 1945, the United States dropped an atomic bomb named “Little Boy” on the Japanese city of Hiroshima, instantly killing tens of thousands of people and leaving lasting devastation. Three days later, a second atomic bomb named “Fat Man” was dropped on Nagasaki, further amplifying the catastrophic destruction. These bombings, with their unprecedented scale of destruction, led to Japan’s surrender and the end of World War II.
The conclusion of World War II marked the beginning of a new geopolitical era characterized by intense ideological tensions between the United States and the Soviet Union. This era, known as the Cold War, witnessed a dangerous escalation in the nuclear arms race between the two superpowers.
The Soviet Union, recognizing the immense power of atomic weapons, embarked on its own nuclear program. Led by Igor Kurchatov and Andrei Sakharov, Soviet scientists made significant strides in nuclear technology, conducting their first successful atomic bomb test in 1949. The arms race between the United States and the Soviet Union heightened fears of a potential nuclear conflict, leading to an era of heightened global anxiety known as the “nuclear standoff.”
Several key events and concepts defined the Cold War era. The term “Mutually Assured Destruction” (MAD) emerged, referring to the concept that both the United States and the Soviet Union possessed enough nuclear firepower to annihilate each other, making any direct conflict catastrophic for both parties. This concept served as a deterrent, ensuring a delicate balance of power.
Tensions reached their peak during the Cuban Missile Crisis in 1962 when the world came dangerously close to nuclear war. The discovery of Soviet missile sites in Cuba, capable of launching nuclear strikes against the United States, led to a tense standoff between President John F. Kennedy and Soviet Premier Nikita Khrushchev. The crisis was eventually resolved through diplomacy and negotiations, averting a catastrophic nuclear conflict.
Nuclear Non-Proliferation and Arms Control Efforts As the Cold War progressed, efforts were made to control the spread of nuclear weapons and reduce the risk of nuclear war. In 1968, the Treaty on the Non-Proliferation of Nuclear Weapons (NPT) was signed, aiming to prevent the proliferation of nuclear weapons while promoting the peaceful use of nuclear energy. The NPT has been instrumental in limiting the number of countries with nuclear capabilities.
Subsequent arms control agreements, such as the Strategic Arms Limitation Talks (SALT) and the Strategic Arms Reduction Treaty (START), aimed to reduce nuclear arsenals and foster trust between the United States and the Soviet Union. These agreements marked important steps in de-escalating the nuclear arms race and reducing the potential for a catastrophic nuclear conflict.
Legacy and Global Implications The Manhattan Project and the subsequent Cold War era left a lasting impact on global politics, scientific research, and public consciousness. The devastating power of atomic weapons, witnessed in the bombings of Hiroshima and Nagasaki, sparked widespread debate on the moral and ethical implications of nuclear warfare.
The emergence of nuclear energy for peaceful purposes also revolutionized various industries, including energy production and medical diagnostics. Nuclear power plants became a source of clean energy, while radiology and nuclear medicine advanced, enabling improved medical imaging and cancer treatments.
Unveiling the Nature of Radiations Emitted by Radioactive Atoms:

The nature of the radiations emitted by radioactive atoms has captivated scientists for over a century. From the pioneering work of Henri Becquerel and Marie Curie to the elucidation of key concepts such as alpha particles, beta particles, and gamma rays, this article provides a comprehensive overview of the nature of radiations and their significance in our understanding of atomic processes.
Discovery of Radioactivity and the Role of Henri Becquerel: The journey into the nature of radiations begins with the accidental discovery of radioactivity by Henri Becquerel, a French physicist. In 1896, while studying uranium salts, Becquerel noticed that these compounds emitted a type of radiation that could penetrate through solid objects. This discovery, made in his laboratory in Paris, laid the foundation for the subsequent exploration of radioactivity.
Alpha Particles: The Nature and Properties: One of the three primary types of radiation emitted by radioactive atoms is alpha particles. These particles consist of two protons and two neutrons, making them identical to the helium nucleus. The discovery and understanding of alpha particles can be attributed to Ernest Rutherford, a New Zealand-born physicist. Rutherford’s experiments with alpha decay revealed that alpha particles possess a positive charge and relatively large mass. Due to their charge and mass, alpha particles have limited penetration power and are easily stopped by a few centimeters of air or a sheet of paper.
Beta Particles: The Nature and Properties: Another form of radiation emitted by radioactive atoms is beta particles. Beta particles can take two forms: beta-minus particles (β-), which are high-energy electrons, and beta-plus particles (β+), which are high-energy positrons. The discovery of beta particles is credited to J.J. Thomson, an English physicist. Thomson’s experiments with cathode rays and beta decay led to the understanding that beta particles are lighter than alpha particles and have a negative charge. Beta particles have greater penetration power compared to alpha particles and can be stopped by a few millimeters of aluminum or plastic.
Gamma Rays: The Nature and Properties: The third type of radiation emitted by radioactive atoms is gamma rays. Gamma rays are a form of electromagnetic radiation, similar to X-rays, but with higher energy. They have no mass or charge and can travel significant distances through matter. The discovery of gamma rays can be attributed to Wilhelm Roentgen, the German physicist who also discovered X-rays. Gamma rays are produced during various processes, including nuclear decay and gamma decay, and possess the highest penetration power among the three types of radiation.
Interaction with Matter and Health Implications: The interactions of alpha particles, beta particles, and gamma rays with matter play a crucial role in determining their effects on living organisms. Alpha particles, due to their large size and positive charge, interact strongly with matter, resulting in a high ionization rate and limited penetration. While this makes alpha particles highly hazardous when inhaled or ingested, their limited penetration power makes them relatively harmless externally.
Beta particles, with their negative charge and lighter mass, can penetrate deeper into matter and cause ionization along their path. This characteristic makes them hazardous both externally and internally, as they can penetrate the skin and tissues, leading to potential health risks.
Gamma rays, being high-energy electromagnetic radiation, possess the highest penetration power among the three types of radiation. They can pass through various materials, including human tissues, with ease. Consequently, gamma rays are highly penetrating and pose a significant health risk when exposed externally or internally.
Understanding the nature of radiations emitted by radioactive atoms is crucial in assessing their potential health implications. Exposure to high levels of radiation can have harmful effects on living organisms, including DNA damage, increased risk of cancer, and radiation sickness. Therefore, strict safety measures and regulations are essential to minimize exposure and protect individuals working with radioactive materials.
Radiation Detection and Protection: The nature of radiations emitted by radioactive atoms necessitates the development of effective detection and protection methods. Various instruments and techniques have been devised to detect and measure the intensity of radiation. Geiger-Muller counters and scintillation detectors are commonly used to measure the presence and intensity of alpha, beta, and gamma radiation.
In terms of protection, shielding materials such as lead, concrete, and water can be used to attenuate the penetration of different types of radiation. The choice of shielding depends on the energy and intensity of the radiation source.
Revealing the Source of Energy: The Nature of Radioactivity

The origin of energy in radioactivity has fascinated scientists for centuries. From the groundbreaking discoveries of pioneers such as Henri Becquerel, Marie Curie, and Ernest Rutherford to the elucidation of key concepts like nuclear decay, atomic nuclei, and binding energy, this comprehensive exploration delves into the mechanisms behind the energy release in radioactive processes.
The Discovery of Radioactivity and the Role of Henri Becquerel: The journey into understanding the nature of radioactivity began with the accidental discovery by Henri Becquerel, a French physicist, in the late 19th century. Becquerel observed that uranium salts emitted radiation capable of penetrating solid objects. This serendipitous finding led to the subsequent exploration of radioactivity and its sources of energy.
Marie Curie and the Radioactive Elements: The pioneering work of Marie Curie, a Polish-born physicist, further expanded our understanding of radioactivity. Curie’s research led to the discovery of polonium and radium, two highly radioactive elements. She established that the energy emitted by these elements originated from within their atomic structures, triggering subsequent investigations into the nature of atomic processes and energy release.
Ernest Rutherford and the Nuclear Model: Ernest Rutherford, a New Zealand-born physicist, made significant contributions to our understanding of atomic structure and the source of energy in radioactivity. Through his famous gold foil experiment, Rutherford discovered that atoms possess a dense, positively charged nucleus at their center, surrounded by negatively charged electrons. This groundbreaking model of the atom explained the concentrated source of energy within atomic nuclei, which is involved in the phenomena of radioactivity.
Nuclear Decay and Energy Release: At the heart of radioactivity lies the process of nuclear decay, which involves the spontaneous transformation of atomic nuclei. Radioactive isotopes, such as uranium, radium, and polonium, undergo decay and release energy in the form of radiation.
There are several types of radioactive decay, including alpha decay, beta decay, and gamma decay. Each type involves specific particles or electromagnetic radiation being emitted from the atomic nucleus. In alpha decay, alpha particles consisting of two protons and two neutrons are emitted. Beta decay involves the release of beta particles, which can be either electrons (beta-minus decay) or positrons (beta-plus decay). Gamma decay emits high-energy gamma rays, a form of electromagnetic radiation.
Binding Energy and the Mass-Energy Equivalence: The source of energy in radioactivity can be traced to the concept of binding energy within atomic nuclei. Binding energy refers to the energy required to hold the nucleus together. According to Albert Einstein’s mass-energy equivalence principle (E=mc²), the mass lost during nuclear decay is converted into energy. This released energy is a result of the difference in binding energy between the initial and final nuclear states.
The significance of this conversion can be seen in the tremendous amount of energy released in nuclear reactions, such as those harnessed in nuclear power and atomic bombs. The mass defect, caused by the difference in mass before and after nuclear decay, is converted into a vast amount of energy, as famously demonstrated by the destructive power of atomic bombs.
Applications and Implications: Understanding the nature of energy release in radioactivity has profound implications across various domains. In nuclear power generation, controlled nuclear reactions harness the energy released through fission or fusion processes to produce electricity. Nuclear power provides a significant source of clean and efficient energy.
However, it is crucial to address the potential hazards associated with radioactivity and the proper management of radioactive materials. Radioactive waste disposal, radiation protection, and stringent safety measures are imperative to safeguard human health and the environment.
Furthermore, the knowledge of radioactivity and its energy sources has far-reaching implications in fields such as nuclear medicine and radiation therapy. Radioactive isotopes are utilized in diagnostic imaging, cancer treatments, and various medical procedures, contributing to advancements in healthcare and disease management.
Research and Advancements: Continued research and advancements in the understanding of radioactivity and its energy sources are ongoing. Scientists strive to unravel the complexities of atomic nuclei, explore new isotopes, and develop innovative techniques for harnessing nuclear energy while minimizing its potential risks.
Unleashing the Power of Radioactivity:
Radioactivity, with its unique properties and energy release, has revolutionized various fields, from medicine to industry and beyond. From the pioneering work of Marie Curie to the modern applications in radiation therapy, radiography, and industrial inspection, this comprehensive exploration delves into the countless ways in which radioactivity has shaped our world.
Medicine and Nuclear Medicine: One of the most significant domains where radioactivity finds extensive use is medicine. The field of nuclear medicine harnesses the power of radioactivity for diagnostic and therapeutic purposes. Radioactive isotopes are used in diagnostic imaging techniques such as positron emission tomography (PET) and single-photon emission computed tomography (SPECT). These techniques allow doctors to visualize and evaluate the functioning of organs and detect diseases at the molecular level.
The pioneering work of George de Hevesy, a Hungarian radiochemist, led to the development of radioisotope tracers. Hevesy introduced radioactive isotopes as tracers to study the metabolism and distribution of various elements within the human body. This technique revolutionized medical research and diagnostics.
Radiation Therapy: Radioactivity plays a crucial role in radiation therapy, a key treatment modality for cancer. Marie Curie and her daughter Irene Joliot-Curie were pioneers in utilizing radioactivity for therapeutic purposes. They developed the concept of radioactive isotopes as sources of radiation for targeted destruction of cancer cells. Today, radiation therapy is an integral part of cancer treatment, with techniques such as external beam radiation and brachytherapy delivering controlled doses of radiation to cancerous tumors.
Radiography and Imaging: Another vital application of radioactivity is in radiography and imaging. Wilhelm Roentgen, a German physicist, discovered X-rays in 1895, opening a new era in medical imaging. X-rays, a form of high-energy electromagnetic radiation, can penetrate the human body and create images that aid in diagnosing fractures, dental issues, and various internal conditions.
Industrial Applications: Radioactivity finds extensive use in various industrial applications, including material inspection and quality control. The ability of radiation to penetrate through objects makes it invaluable in detecting defects, cracks, and inconsistencies in materials. This application is essential in industries such as aerospace, manufacturing, and construction.
Notable Figures and Their Contributions: Several individuals have made significant contributions to the field of radioactivity and its applications. In addition to Marie Curie and Wilhelm Roentgen, other notable figures include:
- Georges Charpak: A French physicist who developed the multi-wire proportional chamber (MWPC), an essential component of modern particle detectors used in nuclear physics, medical imaging, and industrial applications.
- Ernest O. Lawrence: An American physicist who invented the cyclotron, a particle accelerator that revolutionized nuclear physics research and made significant contributions to the development of medical isotopes for diagnosis and therapy.
- Samuel Ruben: An American chemist who co-invented the rubidium-strontium dating method, which uses radioactive decay to determine the age of rocks and minerals, providing valuable insights into geological processes.
- Geraldine Seydoux: An American developmental biologist who utilized radioisotopes to study cell lineage and embryonic development, contributing to our understanding of early development processes.
Radiation Safety and Regulations: While the applications of radioactivity offer numerous benefits, it is essential to maintain stringent safety measures and adhere to regulatory guidelines to ensure the safe handling and disposal of radioactive materials. Regulatory bodies such as the International Atomic Energy Agency (IAEA) and national agencies oversee the use of radioactivity, setting standards for radiation protection and safety.
Proper training, shielding, and monitoring systems are vital to protect workers and the general public from unnecessary exposure to radiation. Strict protocols are followed in the transportation, storage, and disposal of radioactive materials to minimize the potential risks associated with their use.
Ongoing Research and Future Directions: The field of radioactivity continues to evolve with ongoing research and technological advancements. Scientists and researchers are continually exploring new applications and refining existing techniques.
In nuclear medicine, efforts are focused on developing more precise and targeted therapies, such as molecular imaging and theranostics, which combine diagnostics and therapeutics using radioactive isotopes. This approach holds immense potential for personalized medicine, allowing for tailored treatments based on an individual’s unique characteristics.
Advancements in radiation therapy aim to improve treatment efficacy while minimizing side effects. Techniques such as intensity-modulated radiation therapy (IMRT) and proton therapy enable more precise delivery of radiation, sparing healthy tissues and reducing collateral damage.
In the field of material inspection, non-destructive testing using radioactivity continues to advance, with improved imaging techniques and more efficient detection of flaws in materials. This is essential for ensuring the safety and reliability of critical infrastructure and components.
Antoine Henri Becquerel and Radioactivity: A Revolutionary Journey into the Depths of Matter

His groundbreaking work on radioactivity not only transformed our understanding of matter but also laid the foundation for a myriad of scientific advancements that continue to shape our world today. This article delves into the remarkable life and achievements of Becquerel, highlighting his pivotal role in unraveling the mysteries of radioactivity.
Born on December 15, 1852, in Paris, France, Antoine Henri Becquerel hailed from a family renowned for their scientific acumen. He was the grandson of Antoine César Becquerel, a physicist who made significant contributions to the field of electrochemistry. Becquerel’s father, Alexandre-Edmond Becquerel, was also a prominent physicist, specializing in luminescence and phosphorescence.
Becquerel’s exposure to scientific research began at an early age, as he grew up surrounded by his father’s laboratory equipment and academic discussions. In pursuit of his own scholarly path, he attended the prestigious École Polytechnique, where he honed his skills in mathematics and physics. Becquerel’s education was further enriched by the guidance of esteemed scientists such as Gustave-Gaspard de Coriolis and Charles-Augustin de Coulomb, whose influence helped shape his scientific trajectory.
Exploring the Properties of Uranium:
One of the defining moments in Becquerel’s career came when he turned his attention to the study of uranium. Inspired by the works of Wilhelm Conrad Roentgen, the discoverer of X-rays, Becquerel embarked on a mission to understand the peculiar properties of uranium and its potential for emitting radiation. His experiments led him to discover a new form of radiation that he named “uranic rays,” a precursor to the term “radioactivity.”
The Serendipitous Discovery of Radioactivity:
On a fateful day in early 1896, Becquerel stumbled upon a remarkable phenomenon that would forever change the course of science. While conducting experiments with uranium salts, he accidentally left a photographic plate near a sample, believing it would be exposed only to sunlight. However, to his astonishment, the plate was visibly affected despite being shielded from any external light source. This accidental discovery revealed that uranium emitted a type of radiation capable of penetrating solid objects and affecting light-sensitive materials.
Recognizing the significance of his findings, Becquerel sought to further investigate radioactivity with the help of like-minded scientists. This quest for knowledge led him to collaborate with Pierre Curie and Marie Curie, a husband-and-wife team renowned for their work on radioactivity. Together, they embarked on a series of experiments, ultimately leading to the discovery of new radioactive elements such as polonium and radium.
Nobel Prize and Becquerel’s Legacy:
In 1903, Becquerel and the Curies were jointly awarded the Nobel Prize in Physics for their groundbreaking research on radioactivity. This prestigious recognition cemented Becquerel’s status as a pioneering figure in the scientific community. His work not only revolutionized our understanding of atomic structure but also paved the way for significant advancements in fields such as medicine, energy, and nuclear physics.
Beyond his research, Becquerel held several influential positions throughout his career. He served as the Chair of Electrochemistry at the Muséum National d’Histoire Naturelle and was a member of various esteemed scientific societies, including the Académie des Sciences. Becquerel’s contributions to science continued to shape the trajectory of scientific inquiry for years to come.
Becquerel’s discovery of radioactivity opened up a new realm of scientific exploration. It sparked intense interest and inspired countless researchers to delve into the intricacies of atomic and subatomic phenomena. His findings laid the foundation for the field of nuclear physics and propelled advancements in several areas:
- Medical Applications: The study of radioactivity revolutionized medical diagnostics and treatments. Radioactive isotopes, such as iodine-131 and technetium-99m, became essential tools for imaging techniques like positron emission tomography (PET) and single-photon emission computed tomography (SPECT). Radiotherapy, employing controlled doses of radiation, emerged as an effective treatment for various forms of cancer.
- Energy Generation: Becquerel’s work on radioactivity played a pivotal role in the development of nuclear power. The harnessing of nuclear reactions, such as nuclear fission, led to the production of vast amounts of energy. Nuclear power plants became a significant source of electricity generation, offering a cleaner alternative to fossil fuels.
- Fundamental Understanding of Matter: Becquerel’s research shed light on the nature of matter itself. It contributed to the formulation of new atomic models and theories, including Ernest Rutherford’s pioneering work on the atomic nucleus. The exploration of radioactivity unveiled the existence of different types of particles, such as alpha, beta, and gamma radiation, deepening our comprehension of the building blocks of the universe.
- Environmental Impact: The study of radioactivity has also had implications for environmental science. The release of radioactive materials through nuclear accidents, such as the Chernobyl disaster and the Fukushima nuclear accident, highlighted the need for stringent safety measures and reinforced the importance of responsible management of nuclear technology.
Antoine Henri Becquerel’s impact on science transcends his own lifetime. His work laid the groundwork for future generations of scientists to build upon, driving innovation and progress in numerous fields. Becquerel’s contributions continue to inspire scientists to push the boundaries of knowledge, explore new frontiers, and address the challenges and opportunities presented by the atomic world.
The significance of Becquerel’s discoveries is commemorated through the recognition of his name in various scientific contexts. The becquerel (Bq), a unit measuring radioactive decay, was named in his honor. Additionally, the Becquerel Medal, awarded by the French Academy of Sciences, serves as a testament to his enduring legacy.
Marie Curie and Radioactivity: Unraveling the Mysteries of the Atomic World

Marie Curie, born Maria Skłodowska on November 7, 1867, in Warsaw, Poland, was a pioneering scientist whose revolutionary work on radioactivity reshaped the scientific landscape of the early 20th century. Through her groundbreaking discoveries, tireless research, and unwavering dedication, Curie not only became the first woman to win a Nobel Prize but also left an enduring legacy in the fields of physics, chemistry, and medicine.
Marie Curie’s passion for science emerged early in her life. Raised in a family that valued education and intellectual pursuits, she received a rigorous education in Warsaw. However, as opportunities for women to pursue higher education were limited in Poland at the time, Curie embarked on a remarkable journey to further her scientific aspirations.
In 1891, Curie moved to Paris, France, to study at the renowned Sorbonne University. Despite facing numerous challenges as a woman in a predominantly male-dominated field, she excelled in her studies, immersing herself in the world of physics and mathematics. It was during her time at the Sorbonne that Curie met her future husband and collaborator, Pierre Curie, who would play a pivotal role in her scientific journey.
Groundbreaking Research on Radioactivity:
Curie’s fascination with the phenomenon of radioactivity drove her research endeavors. Building upon the work of Antoine Henri Becquerel, who had discovered the phenomenon of radioactivity in uranium, Curie delved into the intricate properties of radioactive elements. Her meticulous experiments and unwavering determination led to the discovery of two new elements: polonium, named after her native Poland, and radium.
The Curie’s groundbreaking research on radioactivity challenged established scientific paradigms, revealing a fundamental property of matter that had eluded scientists until then. They coined the term “radioactivity” to describe the spontaneous emission of radiation by certain elements, fundamentally altering our understanding of the atomic world.
Pioneering Work and Nobel Prizes:
In recognition of their groundbreaking contributions, Marie Curie and her husband, Pierre Curie, jointly received the Nobel Prize in Physics in 1903. This prestigious honor made Marie Curie the first woman ever to be awarded a Nobel Prize. Their discoveries not only revolutionized the field of physics but also laid the foundation for future advancements in medicine, energy, and industry.
Tragedy struck the Curie family in 1906 when Pierre Curie tragically died in a road accident. Despite the immense personal loss, Marie Curie persevered, continuing their scientific work and forging her own path as a leading scientist.
In 1911, Curie received her second Nobel Prize, this time in Chemistry, in recognition of her isolation of pure radium and her contributions to the understanding of its atomic properties. This remarkable achievement made her the first person, and to this day the only woman, to receive Nobel Prizes in two different scientific fields.
Marie Curie’s pioneering research on radioactivity and her relentless pursuit of knowledge had far-reaching implications for science and society. Her discoveries laid the foundation for the development of new fields, such as nuclear physics and radiology, and opened up new avenues for medical diagnostics and treatments.
The practical applications of Curie’s work were instrumental during World War I when she established mobile radiography units, known as “Little Curies,” to assist battlefield surgeons in performing X-rays on wounded soldiers. Her selflessness and dedication to helping others showcased the immense societal impact of her scientific achievements.
Marie Curie’s contributions to science and her trailblazing spirit continue to inspire generations of scientists, particularly women in STEM fields. Her unwavering determination, intellectual curiosity, and groundbreaking discoveries have cemented her as one of the most influential scientists in history.
In addition to her Nobel Prizes, Curie was the first female professor at the University of Paris and became the head of the Radium Institute, which she founded. She was a founding member of the International Atomic Weights Committee and played a crucial role in shaping the emerging field of radioactivity research.
Curie’s impact is immortalized in various honors and awards. The Curie Institute in Paris, an internationally renowned research center, bears her name, as does the radioactive element “curium.” Additionally, the Marie Curie Actions, part of the European Union’s Horizon 2020 program, provide funding and support for researchers across Europe.
Marie Curie’s remarkable achievements came amidst numerous challenges and prejudices. As a woman in a male-dominated field, she faced discrimination and skepticism, yet she persevered, breaking barriers and proving her scientific prowess.
Curie’s work with radioactive materials also took a toll on her health. The long-term exposure to radiation eventually led to her premature death on July 4, 1934, at the age of 66. However, her legacy lives on, not only through her scientific accomplishments but also through her advocacy for education, gender equality, and the pursuit of scientific knowledge.
Ernest Rutherford and Radioactivity: Pioneering Discoveries and Impact on Science

Ernest Rutherford, a renowned New Zealand-born physicist, made groundbreaking contributions to the understanding of radioactivity and nuclear physics in the early 20th century. His tireless research and innovative experiments revolutionized our understanding of the atom, leading to the development of new theories and technologies that continue to shape the world of science.
Ernest Rutherford was born on August 30, 1871, in Nelson, New Zealand. Growing up in a modest family, he showed exceptional academic abilities from a young age. Rutherford’s interest in science led him to attend Nelson College, where he excelled in mathematics and physics. In 1890, he received a scholarship to study at the University of New Zealand, which marked the beginning of his remarkable scientific journey.
Key Discoveries in Radioactivity:
Rutherford’s first significant breakthrough came while he was conducting experiments with radioactivity at the University of Cambridge. In collaboration with his mentor J.J. Thomson, Rutherford investigated the nature of radiation emitted by uranium compounds. In 1899, they discovered two distinct types of radiation: alpha particles and beta particles. Rutherford went on to propose a third type, which he called gamma radiation.
In 1902, Rutherford made a critical observation during his study of uranium radiation. He noticed that the emissions consisted of both charged particles (later identified as alpha and beta particles) and uncharged particles. This led him to hypothesize the existence of a neutral, uncharged particle within the atom, which he termed the “neutron.” Although Rutherford’s concept of the neutron differed from the modern understanding, his initial insight paved the way for future discoveries.
Rutherford’s most famous experiment, the gold foil experiment, was conducted in 1909 at the University of Manchester. In this experiment, Rutherford bombarded a thin gold foil with alpha particles emitted from a radioactive source. He expected the alpha particles to pass through the gold foil with minimal deflection, as per the prevailing Thomson atomic model.
However, Rutherford’s observations during the gold foil experiment were surprising and transformative. Contrary to his expectations, he found that some alpha particles were deflected at large angles, and a few even bounced back directly. This led Rutherford to conclude that the atom was mostly empty space, with a tiny, dense, positively charged nucleus at its center.
Rutherford’s revolutionary insight, known as the nuclear model of the atom, laid the foundation for modern atomic theory. He proposed that the positively charged nucleus contained most of the atom’s mass, while the negatively charged electrons orbited around it in specific energy levels. This model provided a more accurate description of atomic structure and explained the behavior of radiation and the stability of atoms.
Rutherford’s contributions to radioactivity extended beyond theoretical models. He also conducted experiments to explore the concept of radioactive decay and the transmutation of elements. Through his studies, he discovered that radioactive elements spontaneously underwent decay, transforming into other elements while emitting radiation. This discovery challenged the notion that elements were immutable and opened up avenues for further research on nuclear transformations.
In 1919, Rutherford achieved another significant milestone by artificially inducing the first nuclear reaction. Collaborating with his colleagues, Hans Geiger and Ernest Marsden, he bombarded nitrogen gas with alpha particles, leading to the creation of oxygen and hydrogen nuclei. This groundbreaking experiment marked the first human-controlled nuclear transformation and demonstrated the potential of manipulating atomic nuclei.
Rutherford’s groundbreaking work in radioactivity earned him numerous accolades and recognition. He was awarded the Nobel Prize in Chemistry in 1908 for his investigations into the disintegration of the elements and the chemistry of radioactive substances. Throughout his career, Rutherford held prestigious positions at renowned institutions such as the University of Manchester, the University of Cambridge, and the Cavendish Laboratory.
The impact of Rutherford’s discoveries on science and technology cannot be overstated. His nuclear model of the atom paved the way for further developments in quantum mechanics, nuclear physics, and the understanding of fundamental particles. Rutherford’s research provided the basis for the development of nuclear energy, nuclear medicine, and the field of particle physics.
Pierre Curie and Radioactivity: Unveiling the Mysteries of Nature’s Power

In the realm of scientific discovery, few names are as prominent as Pierre Curie, a French physicist and pioneer in the field of radioactivity. Alongside his wife, Marie Curie, Pierre Curie made groundbreaking contributions to the understanding of this enigmatic phenomenon. Through their relentless dedication and ingenious experiments, the Curies unlocked the secrets of radioactivity, forever transforming our perception of the atomic world.
Born on May 15, 1859, in Paris, France, Pierre Curie displayed an early aptitude for scientific pursuits. He enrolled at the École Polytechnique, a renowned institution for scientific education, where he delved into various fields of study, including mathematics and physics. Curie’s thirst for knowledge propelled him to further his education at the Sorbonne in Paris, where he earned his doctorate in 1895.
Radioactivity and the Curie’s Collaborative Efforts:
Pierre Curie’s path to scientific greatness intersected with that of Marie Skłodowska, who would later become his wife. Together, they embarked on a shared scientific journey that focused on the recently discovered phenomenon of radioactivity. Inspired by the work of Henri Becquerel, the Curies began investigating the properties of radioactive materials.
In 1898, Pierre and Marie Curie, alongside Henri Becquerel, discovered a new radioactive element, which they named polonium after Marie’s native Poland. This groundbreaking finding propelled the Curies into the scientific limelight and marked the beginning of their profound contributions to the field of radioactivity.
The Curies’ relentless research led them to another significant discovery in the same year. They isolated a second radioactive element, which they named radium, owing to its intense radiation properties. Pierre Curie, with his remarkable experimental skills, developed innovative techniques to extract and purify these radioactive elements, enabling further exploration into their properties.
Pierre Curie made invaluable contributions to the study of radioactivity beyond the discovery of new elements. He played a crucial role in developing the concept of radioactive decay, wherein unstable atomic nuclei undergo spontaneous transformations, emitting radiation in the process. Curie’s mathematical insights and meticulous observations allowed for a deeper understanding of this fundamental process.
Furthermore, Pierre Curie’s rigorous scientific approach and meticulous measurements laid the groundwork for future research on the nature of radiation. His studies on the thermoelectric effects of radioactive substances provided essential data that contributed to the development of thermoelectric power generators and other practical applications.
Tragically, Pierre Curie’s life was cut short on April 19, 1906, when he died in a street accident in Paris. However, his groundbreaking research and his unwavering dedication to the study of radioactivity continue to resonate to this day.
Pierre Curie’s legacy extends far beyond his lifetime. His research laid the foundation for subsequent advancements in nuclear physics and atomic theory. The Curies’ work not only unveiled the complexities of radioactivity but also revolutionized our understanding of the atom itself. Their discoveries shattered conventional scientific beliefs and opened new avenues for research, leading to the birth of nuclear science and its applications in energy production, medicine, and industry.
In recognition of their monumental contributions, Pierre and Marie Curie were jointly awarded the Nobel Prize in Physics in 1903, becoming the first couple to receive this prestigious honor.
Wilhelm Röntgen and the Discovery of X-Rays:

A German physicist, Wilhelm Röntgen revolutionary discovery of this mysterious form of radiation in 1895 paved the way for a new era in medical diagnostics and scientific exploration.Born on March 27, 1845, in Lennep, Prussia (now part of Germany), Wilhelm Röntgen displayed an early fascination with the natural sciences. He pursued his education at the University of Utrecht in the Netherlands, where he studied mechanical engineering before shifting his focus to physics. Röntgen’s determination and aptitude led him to excel in his studies, laying the foundation for his future scientific endeavors.
The Discovery of X-Rays:
In 1895, while conducting experiments at the University of Würzburg in Germany, Wilhelm Röntgen stumbled upon a mysterious phenomenon that would revolutionize the scientific world. Working with a cathode ray tube, he noticed that a screen coated with fluorescent material in his lab began to emit a faint, greenish glow even when shielded from direct contact with the cathode rays.
Intrigued by this unexpected observation, Wilhelm Röntgen meticulously investigated this newfound radiation. He labeled it “X-rays,” using the mathematical symbol “X” to signify its unknown nature. Röntgen soon discovered that these rays possessed remarkable properties—they could penetrate various substances, including human tissue, and produce shadow-like images on photographic plates.
Wilhelm Röntgen groundbreaking experiments led to several crucial discoveries. He found that X-rays could pass through materials such as wood and paper but were partially absorbed by denser substances like bone and metal. This property made X-rays immensely valuable for medical imaging, allowing doctors to visualize internal structures and diagnose ailments without invasive procedures.
In 1896, Wilhelm Röntgen captured the first-ever X-ray image—an image of his wife’s hand, revealing the skeletal structure and the presence of her wedding ring. This iconic image showcased the potential of X-rays in the field of medicine and spurred a wave of enthusiasm for their practical applications.
Wilhelm Röntgen meticulous observations and systematic approach also led him to make significant contributions to the understanding of X-rays. He discovered that X-rays were not deflected by electric or magnetic fields, suggesting that they were not composed of charged particles. Additionally, Wilhelm Röntgen found that X-rays were produced when high-speed electrons struck a metal target, a phenomenon known as X-ray fluorescence.
Wilhelm Röntgen discovery of X-rays revolutionized medical diagnostics and profoundly impacted various scientific disciplines. The use of X-rays became widespread in medicine, enabling doctors to visualize internal injuries, detect diseases, and guide surgical interventions. The field of radiology emerged, and X-ray technology rapidly advanced, leading to the development of more sophisticated imaging techniques such as computed tomography (CT) scans and mammography.
Wilhelm Röntgen contribution to the field of radioactivity earned him numerous accolades, including the first Nobel Prize in Physics in 1901. His discovery marked the birth of a new era in scientific research, propelling further investigations into the nature of radiation and atomic structure. The exploration of X-rays paved the way for subsequent breakthroughs, such as the discovery of radioisotopes, radiation therapy for cancer treatment, and advancements in nuclear physics and imaging technologies.
Wilhelm Röntgen discovery also sparked global interest and spurred scientific advancements beyond medicine. Researchers began exploring the properties of X-rays in various fields, including material science, archaeology, and astronomy. X-ray crystallography, a technique that uses X-rays to study the atomic and molecular structure of crystals, became a powerful tool for understanding the arrangement of atoms in matter.
Furthermore, Wilhelm Röntgen work on X-rays inspired a new wave of scientific inquiry into the nature of radiation itself. His groundbreaking discovery prompted further investigations into the properties of electromagnetic waves and the electromagnetic spectrum, expanding our understanding of the fundamental forces that govern the universe.
Wilhelm Röntgen legacy as a pioneer of radioactivity and X-rays lives on. His name has become synonymous with the field, as the unit of exposure to ionizing radiation, the “Wilhelm Röntgen,” is named in his honor. The impact of his discovery on society is immeasurable, as X-ray technology has become an indispensable tool in healthcare, research, and industry.
Edmond Becquerel and the Pioneering Studies in Radioactivity

A French physicist, Becquerel made pioneering contributions to the understanding of this phenomenon, laying the foundation for future advancements in the field. Edmond Becquerel was born on March 24, 1820, in Paris, France, into a family with a rich scientific heritage. His father, Antoine César Becquerel, and grandfather, Alexandre-Edmond Becquerel, were both esteemed physicists, renowned for their work in electricity and luminescence. Growing up in this scientific environment, Edmond Becquerel developed a passion for scientific inquiry from an early age.
Following in the footsteps of his family, Becquerel pursued a career in scientific research. He dedicated his early studies to luminescence, the emission of light by certain materials, and its relationship to photochemistry. He conducted numerous experiments to investigate the phenomenon of phosphorescence and developed innovative instruments for measuring light emissions.
Becquerel’s Breakthrough in Radioactivity:
In 1896, at the age of 76, Becquerel made a groundbreaking discovery while exploring the properties of uranium salts. Inspired by the recent findings of Wilhelm Röntgen on X-rays, he sought to determine if phosphorescent materials, such as uranium, emitted similar rays. In his experiments, Becquerel observed that uranium salts spontaneously produced a form of radiation that could penetrate opaque materials.
This accidental discovery marked the birth of the field of radioactivity. Becquerel realized that certain elements emitted radiation without the need for external stimulation, making them inherently radioactive. His work laid the foundation for further investigations into the nature of radioactivity and its implications.
Becquerel’s groundbreaking discovery attracted the attention of fellow scientists, including Marie Curie and her husband Pierre Curie. The Curies built upon Becquerel’s research, leading to their own groundbreaking discoveries in radioactivity and the subsequent isolation of polonium and radium.
In recognition of his significant contributions to the field, Edmond Becquerel was elected to the French Academy of Sciences in 1889 and received numerous awards and honors throughout his career. However, despite his remarkable achievements, Becquerel remained a modest and dedicated scientist, continuously pursuing further investigations into radioactivity.
Edmond Becquerel’s pioneering studies in radioactivity played a crucial role in shaping the development of the field. His accidental discovery opened up new avenues of scientific inquiry and set the stage for subsequent advancements by future researchers.
Becquerel’s work not only laid the groundwork for the discoveries made by the likes of the Curies but also paved the way for future breakthroughs in nuclear physics, leading to the unraveling of the atom’s structure and the development of nuclear energy.
The impact of Becquerel’s research extends beyond the realm of scientific knowledge. Radioactivity and its applications have found numerous practical uses, ranging from medical diagnostics and treatments to energy production and industrial applications.
Antoine César Becquerel:

A French physicist, Becquerel made significant contributions to the understanding of this phenomenon, paving the way for future advancements in the field. Antoine César Becquerel was born on March 7, 1788, in Châtillon-sur-Loing, France. He hailed from a family with a rich scientific heritage and grew up in an environment steeped in intellectual curiosity. His father, Alexandre-Théophile Becquerel, was a renowned scientist who conducted important research in electricity and optics.
Becquerel’s career followed in his father’s footsteps, and he became a prominent physicist in his own right. His scientific endeavors covered various fields, including electricity, optics, and photoluminescence. However, it was his pioneering work in the study of radioactivity that solidified his place in scientific history.
Explorations in Radioactivity:
Becquerel’s interest in radioactivity was sparked by the discovery of X-rays by Wilhelm Röntgen in 1895. Inspired by this new area of inquiry, Becquerel began investigating the phenomenon of phosphorescence, the emission of light by certain substances after exposure to radiation.
In his experiments, Becquerel focused on the phosphorescent properties of uranium salts. He hypothesized that these salts might emit radiation that was similar to X-rays. To test this hypothesis, Becquerel exposed photographic plates to uranium salts and observed their behavior.
Becquerel’s experiments yielded significant results. He discovered that uranium salts emitted a form of radiation that could penetrate opaque materials and affect photographic plates, even when shielded from external sources of light. This phenomenon, which he called uranium rays, marked the first identification of natural radioactivity.
Becquerel’s investigations also led to the discovery of the unique properties of beta radiation emitted by radioactive substances. He observed that this type of radiation was deflected by magnetic fields, suggesting that it consisted of charged particles. This observation paved the way for future research on the nature of radioactivity.
Antoine César Becquerel’s pioneering studies in radioactivity earned him recognition and respect within the scientific community. He was awarded the Copley Medal by the Royal Society of London in 1853 for his contributions to the field of physics.
Becquerel’s groundbreaking work laid the foundation for future advancements in the study of radioactivity. His discoveries set the stage for the groundbreaking research of his son, Alexandre-Edmond Becquerel, and his grandson, Henri Becquerel, who would go on to discover the phenomenon of radioactive decay and share the 1903 Nobel Prize in Physics with Marie and Pierre Curie.
Irène Joliot-Curie and the Legacy of Radioactivity:

In the realm of scientific achievement, the name Irène Joliot-Curie shines brightly as a trailblazer in the field of radioactivity. As the daughter of Marie Curie and Pierre Curie, Irène Joliot-Curie continued her family’s groundbreaking work and made her own indelible mark on the study of atomic particles.
Irène Joliot-Curie was born on September 12, 1897, in Paris, France, into a family of scientific luminaries. Her parents, Marie and Pierre Curie, were Nobel laureates known for their revolutionary research on radioactivity. Growing up in an environment that nurtured intellectual curiosity, Joliot-Curie developed a passion for science from an early age.
Inspired by her parents’ work, Joliot-Curie pursued her education at the University of Paris, where she studied mathematics and physics. Under the tutelage of renowned physicists, she honed her scientific skills and delved into the mysteries of atomic structure and radioactivity.
Collaboration and Groundbreaking Discoveries:
Joliot-Curie’s scientific journey was intrinsically linked to her collaboration with her husband, Frédéric Joliot. Together, they conducted pioneering research in the field of nuclear physics, with a focus on the artificial production of radioactive isotopes.
In 1934, Joliot-Curie and her husband achieved a significant breakthrough. They bombarded aluminum with alpha particles, leading to the creation of a radioactive isotope of phosphorus. This groundbreaking experiment marked the first artificial production of a radioactive element and demonstrated the possibility of transmuting elements through nuclear reactions.
Their remarkable achievement led to the discovery of artificial radioactivity, a phenomenon where stable elements become radioactive when bombarded with particles. Joliot-Curie and her husband continued to explore this new avenue of research, artificially producing several other radioactive isotopes and deepening our understanding of nuclear reactions.
In recognition of their groundbreaking work, Irène Joliot-Curie and Frédéric Joliot were awarded the Nobel Prize in Chemistry in 1935, becoming the first married couple to receive a Nobel Prize. This prestigious accolade highlighted the significance of their contributions to the field of radioactivity.
Joliot-Curie’s scientific legacy extended beyond her groundbreaking discoveries. She played a crucial role in establishing the Curie Institute, a renowned research institution dedicated to the study of radioactivity and cancer treatment. As the director of the institute’s Radium Laboratory, she continued to push the boundaries of scientific knowledge and mentored numerous aspiring scientists.
Irène Joliot-Curie’s unwavering dedication to scientific research and her invaluable contributions to the field of radioactivity continue to inspire generations of scientists. Her pioneering work paved the way for advancements in nuclear physics, artificial radioisotope production, and the broader understanding of atomic structure.
Frederick Soddy and the Study of Radioactivity:

In the realm of scientific exploration, the name Frederick Soddy stands tall as a pioneering figure in the study of radioactivity. As a British chemist and Nobel laureate, Soddy made significant contributions to our understanding of atomic structure, radioactive decay, and the concept of isotopes. Frederick Soddy was born on September 2, 1877, in Eastbourne, England. He displayed a keen interest in science from an early age and pursued his education at the University of Oxford. Soddy’s academic pursuits led him to delve into various scientific disciplines, including chemistry and physics, setting the stage for his future scientific endeavors.
Collaboration with Ernest Rutherford:
Soddy’s career path crossed with that of another luminary in the field, Ernest Rutherford, during his time at the University of Glasgow. The collaboration between Soddy and Rutherford would prove to be pivotal in the study of radioactivity.
One of their notable joint achievements was the concept of radioactive decay. Building upon the work of Henri Becquerel, they proposed that certain elements spontaneously undergo transformations, emitting radiation in the process. This groundbreaking insight challenged the prevailing belief that elements were immutable and set the stage for further investigations into atomic transmutation.
Soddy’s Pioneering Work on Isotopes:
In 1910, Soddy made a pivotal discovery that would earn him lasting acclaim. Through his investigations into radioactive decay, he recognized that different elements could have multiple forms with identical chemical properties but differing atomic weights. He termed these variants isotopes, a term that would become fundamental to the understanding of atomic structure.
Soddy’s work on isotopes laid the foundation for further advancements in nuclear chemistry and provided a new perspective on the behavior of elements. His insights into isotopic transformation and radioactive decay significantly contributed to our understanding of the stability and transmutation of atomic nuclei.
Frederick Soddy’s contributions to the study of radioactivity earned him numerous accolades, including the Nobel Prize in Chemistry in 1921. The prize acknowledged his groundbreaking research on isotopes and his role in advancing our understanding of atomic structure and radioactive decay.
Soddy’s scientific legacy extends beyond his own discoveries. His groundbreaking work on isotopes and radioactive decay paved the way for further research in nuclear physics, nuclear medicine, and the development of atomic energy. The concepts he elucidated continue to underpin our understanding of the atomic world and have had far-reaching implications in fields ranging from medical imaging to environmental science.
Key Dates of Radioactivity:

1896: Henri Becquerel discovers natural radioactivity, accidentally observing the ability of uranium salts to expose photographic plates, leading to the birth of the field of radioactivity.
1898: Marie Curie and Pierre Curie discover and isolate two new radioactive elements, polonium and radium, opening the door to further research on radioactivity.
1899: Ernest Rutherford and J.J. Thomson identify two types of radiation emitted by uranium compounds, distinguishing them as alpha particles and beta particles.
1902: Rutherford proposes the existence of a neutral, uncharged particle within the atom, later known as the “neutron,” expanding our understanding of atomic structure.
1903: Marie Curie becomes the first woman to be awarded the Nobel Prize in Physics, recognizing her outstanding contributions to the field of radioactivity.
1909: Rutherford conducts the famous gold foil experiment at the University of Manchester, revealing the existence of a dense, positively charged atomic nucleus.
1911: Rutherford formulates the nuclear model of the atom, proposing that electrons orbit a central nucleus, revolutionizing our understanding of atomic structure.
1913: Niels Bohr introduces his revolutionary Bohr model of the atom, incorporating principles of quantum mechanics and explaining atomic energy levels.
1920: Frederick Soddy introduces the concept of isotopes, recognizing that different atomic forms of an element have identical chemical properties but varying atomic weights.
1932: James Chadwick discovers the neutron, confirming Rutherford’s earlier hypothesis and completing the triad of fundamental subatomic particles.
1934: Irène Joliot-Curie and Frédéric Joliot achieve the first artificial production of a radioactive element, phosphorus, by bombarding aluminum with alpha particles.
1945: The Manhattan Project successfully harnesses the power of nuclear fission, leading to the development of the atomic bomb and ushering in the atomic age.
1952: Linus Pauling proposes the concept of biochemical clocks based on the radioactive decay of specific isotopes, revolutionizing radiometric dating techniques.
1968: Georges Charpak invents the multiwire proportional chamber, a highly sensitive particle detector used in nuclear and particle physics experiments.
1986: The Chernobyl disaster occurs in Pripyat, Ukraine, resulting in a catastrophic nuclear accident and highlighting the potential dangers of radioactive materials.
2012: The Large Hadron Collider (LHC) at CERN confirms the existence of the Higgs boson, shedding light on the mechanism of mass generation in the universe.
Conclusion:
The discovery of radioactivity has been a collaborative effort involving multiple pioneering scientists, each making significant contributions to unraveling the mysteries of atomic phenomena. From the accidental observation of Henri Becquerel in 1896 to the groundbreaking research of Marie and Pierre Curie, Ernest Rutherford, Frederick Soddy, and others, the journey of discovering radioactivity has been a testament to human curiosity and scientific exploration.
Henri Becquerel’s accidental discovery of natural radioactivity while studying uranium salts set the stage for further investigations into this fascinating phenomenon. Marie and Pierre Curie’s relentless research and subsequent isolation of new radioactive elements, polonium and radium, solidified radioactivity as a distinct field of study.
Ernest Rutherford’s experiments and his famous gold foil experiment revealed the existence of the atomic nucleus, transforming our understanding of atomic structure and paving the way for the nuclear model of the atom. Frederick Soddy’s work on isotopes expanded our comprehension of atomic behavior and radioactive decay, laying the foundation for the development of nuclear chemistry.
These remarkable discoveries were complemented by the scientific endeavors of other luminaries, such as Niels Bohr, James Chadwick, Irène Joliot-Curie, Frédéric Joliot, and Linus Pauling, among many others. Their contributions expanded our understanding of subatomic particles, artificial radioactivity, biochemical clocks, and the applications of radioactivity in various fields.
It is crucial to recognize that scientific progress is often a collective endeavor. The discoveries and advancements made by these scientists built upon the work of their predecessors and paved the way for future generations of researchers. Each breakthrough, experiment, and theoretical insight added another piece to the intricate puzzle of radioactivity, bringing us closer to a comprehensive understanding of the atomic world.
In conclusion, radioactivity was not the result of a single discovery but rather a culmination of the efforts of numerous scientists who dedicated their lives to unraveling the secrets of the atomic realm. The discoveries made by Becquerel, the Curies, Rutherford, Soddy, and others have fundamentally transformed our understanding of the atom, radiation, and nuclear reactions. Their collective contributions have had a profound impact on a wide range of fields, from medicine and energy production to fundamental particle physics, leaving an indelible mark on the scientific community and society as a whole.
Reference List:
- Einstein, A. (1905). On the Electrodynamics of Moving Bodies.
- Fermi, E. (1934). Radioactivity Produced by Neutron Bombardment: Nobel Lecture.
- Geiger, H. (1908). On the Scattering of α-Particles by Matter.
- Joliot-Curie, I. (1935). Artificial Production of Radioactive Substances: Nobel Lecture.
- Marsden, E. (1911). On a Diffuse Reflection of the α-Particles.
- Meitner, L. (1939). Disintegration of Uranium by Neutrons: A New Type of Nuclear Reaction.
- Pauling, L. (1948). The Nature of the Chemical Bond.
- Planck, M. (1900). Zur Theorie des Gesetzes der Energieverteilung im Normalspektrum.
- Rutherford, E. (1911). The Scattering of α and β Particles by Matter and the Structure of the Atom.
- Soddy, F. (1913). The Radio-Elements and the Periodic Law.
- Thomson, J. J. (1897). Cathode Rays.
- Van der Waals, J. D. (1873). Over de Continuïteit van
- Wigner, E. P. (1937). On the Possibility of a Metallic Modification of Hydrogen.
- Wolfram, S. (1984). The Mathematica Book.
- Ziegler, J. F. (1985). SRIM – The Stopping and Range of Ions in Matter.