The discovery of the proton, a subatomic particle that plays a fundamental role in the structure of matter, is a testament to the remarkable achievements of scientists who sought to understand the building blocks of the universe. In this extensive article, we delve into the captivating story of the discovery of the proton, exploring the scientists, experiments, and key milestones that led to our understanding of this fundamental particle. Join us on a journey through time and scientific inquiry as we uncover the fascinating world of atomic structure.

The quest to understand the nature of atoms began in the late 19th century with the groundbreaking work of scientists such as J.J. Thomson, Ernest Rutherford, and Eugen Goldstein. These pioneers conducted experiments to explore the properties of cathode rays, the mysterious beams of particles emitted by cathode ray tubes.
In 1897, J.J. Thomson’s experiments with cathode rays led to the discovery of the electron, a negatively charged subatomic particle. Thomson’s observation of the deflection of cathode rays in electric and magnetic fields provided crucial evidence for the existence of these tiny particles within atoms.
Building on Thomson’s work, Ernest Rutherford conducted the famous gold foil experiment in 1911. By bombarding a thin gold foil with alpha particles, Rutherford and his team observed unexpected deflections of some particles, leading to the revolutionary conclusion that atoms possessed a dense, positively charged core known as the nucleus.
The Discovery of the Proton
In 1919, Ernest Rutherford and his colleagues, Hans Geiger and Ernest Marsden, conducted further experiments that shed light on the nature of atomic structure. Their experiments involved bombarding nitrogen gas with alpha particles. Surprisingly, they observed that some of the alpha particles were deflected at larger angles than expected, indicating the presence of a positively charged particle within the nitrogen atom.
Rutherford proposed that this particle was a fundamental building block of matter, which he named the proton. The discovery of the proton revolutionized our understanding of atomic structure, revealing that the nucleus of an atom consists of positively charged protons and neutral or negatively charged electrons orbiting around it.
The existence of the proton was further confirmed by subsequent experiments conducted by scientists such as James Chadwick and Sir Lawrence Bragg. Chadwick’s experiments in the 1930s demonstrated the existence of another fundamental particle, the neutron, which exists alongside protons in the atomic nucleus.
The discovery of the proton opened up new avenues of research and led to significant advances in our understanding of the physical world. The study of subatomic particles, their interactions, and the forces that govern them became central to the field of particle physics. Scientists, using powerful particle accelerators and detectors, have further explored the properties and behaviors of protons and other subatomic particles, contributing to our understanding of the fundamental laws of nature.
The discovery of the proton stands as a landmark achievement in our exploration of the microscopic world. Through the diligent work of scientists such as Rutherford, Thomson, and their contemporaries, we have gained profound insights into the structure of matter and the nature of atoms.
The proton, as a key component of atomic nuclei, plays a vital role in the formation of elements and the interactions between particles. Its discovery paved the way for further breakthroughs in nuclear physics, particle physics, and our understanding of the fundamental forces of the universe.
What is a Proton?

This positively charged particle, residing within the atomic nucleus, is a fundamental building block of matter. In this comprehensive article, we delve into the intricacies of the proton, exploring its properties, discovery, and role in the structure of atoms. Join us on a captivating journey through the subatomic world as we unravel the mysteries of the proton.
The study of atomic structure began in the late 19th century with the groundbreaking experiments of scientists such as J.J. Thomson, Ernest Rutherford, and Niels Bohr. Their pioneering work paved the way for unraveling the secrets of the atom and the role of the proton within it.
The Proton’s Charge and Mass
The proton carries a positive electric charge of +1 elementary charge, denoted as e. Its mass is approximately 1.67 x 10^-27 kilograms. As one of the three primary subatomic particles (alongside electrons and neutrons), protons play a crucial role in the overall electrical charge and stability of atoms.
The discovery of the proton can be attributed to the pioneering work of scientists such as Ernest Rutherford, Hans Geiger, and Ernest Marsden. Through their experiments in the early 20th century, they observed the deflection of alpha particles and deduced the existence of a positively charged particle within the atomic nucleus.
The nucleus of an atom comprises protons and neutrons. Each element is characterized by its unique proton count, which determines its atomic number. For example, hydrogen has a single proton, while helium has two protons. The periodic table provides a systematic organization of elements based on their proton count.
Role of Protons in Atomic Stability
Protons play a vital role in determining the stability of atoms. The positive charge of the protons is balanced by the negatively charged electrons orbiting around the nucleus. This delicate balance of charges ensures the overall neutrality of the atom. The number of protons also affects the chemical properties of elements and determines their behavior in chemical reactions.
Protons and the Strong Nuclear Force
The protons in the nucleus are bound together by the strong nuclear force, one of the four fundamental forces in nature. This force acts to overcome the repulsive electrostatic forces between the positively charged protons, keeping the nucleus stable. The balance between the attractive strong nuclear force and the repulsive electromagnetic force is crucial for maintaining atomic integrity.
Protons not only play a fundamental role in atomic structure but also feature prominently in the field of particle physics. Particle accelerators, such as the Large Hadron Collider (LHC), are used to study the behavior of protons at incredibly high energies. Collisions between protons in these experiments provide insights into the fundamental particles and forces that govern the universe.
Protons find practical applications in various fields. In medicine, proton therapy is a specialized form of radiation treatment used to target and destroy cancerous cells while minimizing damage to healthy tissue. Protons are also utilized in scientific research, such as proton spectroscopy, to study the structure and behavior of molecules.
Early Theories of the Proton:

The ancient Greek philosophers Democritus and Leucippus were among the first to propose the existence of indivisible particles called atoms. However, it was not until the modern era that theories about specific subatomic particles, including the proton, began to emerge.
In the late 19th century, British physicist J.J. Thomson proposed the plum pudding model of the atom. According to this model, the atom was composed of a positively charged material with negatively charged corpuscles (later known as electrons) embedded within it. However, Thomson did not explicitly identify a specific particle as the carrier of positive charge within the atom.
Ernest Rutherford’s experiments in the early 20th century led to the development of the nuclear model of the atom. Through his gold foil experiment, Rutherford discovered that atoms consisted of a tiny, dense, positively charged nucleus surrounded by a cloud of negatively charged electrons. However, Rutherford’s model did not yet explicitly define the nature of the positively charged particles within the nucleus.
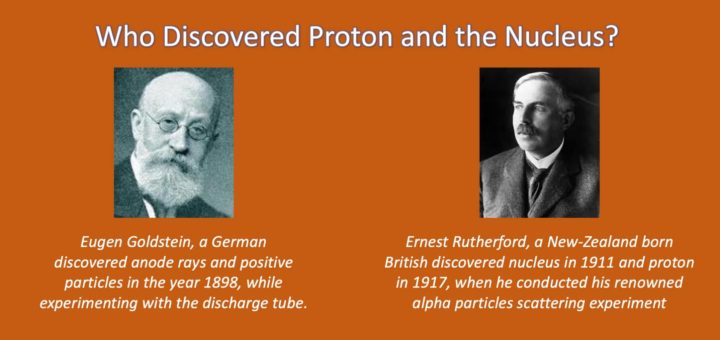
Around the same time, German physicist Eugen Goldstein conducted experiments using cathode rays. He observed particles moving in the opposite direction of the negatively charged electrons, suggesting the presence of positively charged particles within the cathode ray tube. These particles, known as Canal Rays or Goldstein Rays, were the first indication of the existence of positively charged particles in atoms.
The term “proton” was introduced by Ernest Rutherford in 1920. While conducting experiments involving the scattering of alpha particles, Rutherford noticed that some particles were deflected at large angles, indicating the presence of a massive, positively charged particle within the nucleus. Rutherford named this particle the “proton,” derived from the Greek word “protos” meaning “first” or “primary.”
In 1932, British physicist James Chadwick made a groundbreaking discovery that complemented the understanding of the proton. Through his experiments, Chadwick identified the neutron, a particle with no charge, residing alongside protons in the atomic nucleus. This discovery completed the picture of the atomic nucleus, providing a more comprehensive understanding of subatomic particles.
Quantum Mechanics and the Proton
The advent of quantum mechanics in the early 20th century further refined our understanding of the proton. Scientists such as Werner Heisenberg and Erwin Schrödinger developed mathematical frameworks to describe the behavior of subatomic particles, including protons, within the realm of quantum physics. These theories provided a deeper insight into the properties and interactions of protons and other subatomic particles.
Ernest Rutherford and His Proton Discovery:

Sir Ernest Rutherford, a prominent New Zealand-born physicist, made groundbreaking contributions to the field of atomic physics. One of his most significant achievements was the discovery of the proton, a fundamental subatomic particle. In this extensive article, we delve into Rutherford’s life, his innovative experiments, and the profound implications of his proton discovery. Join us on a captivating journey as we explore the scientific legacy of this remarkable scientist.
Ernest Rutherford was born on August 30, 1871, in Brightwater, New Zealand. He demonstrated an early aptitude for science and pursued higher education at the University of Canterbury and the University of Cambridge. Under the mentorship of renowned physicist J.J. Thomson, Rutherford honed his experimental skills and began his exploration of the mysteries of atomic structure.
In 1909, while conducting his famous gold foil experiment at the University of Manchester, Rutherford made a groundbreaking discovery that would reshape our understanding of atomic structure. By directing a beam of alpha particles at a thin gold foil, Rutherford and his colleagues, Hans Geiger and Ernest Marsden, observed unexpected results.
Contrary to their expectations, a small fraction of the alpha particles were deflected at large angles, while the majority passed straight through the foil. Rutherford deduced that the positive charge of the atom must be concentrated in a small, dense central region within the atom, which he named the nucleus.
Building on the findings of his gold foil experiment, Rutherford embarked on further investigations to identify the nature of the positive charge within the atomic nucleus. In 1919, he proposed the existence of a subatomic particle carrying a positive charge, which he named the proton.
Rutherford’s ingenious experiments and meticulous analysis allowed him to determine the charge-to-mass ratio of the proton and estimate its size. Through his innovative techniques, he established the proton as an essential component of atomic structure, alongside the negatively charged electron.
Rutherford’s proton discovery revolutionized our understanding of the atom. His model of the atom, known as the Rutherford model, portrayed an atom with a tiny, positively charged nucleus at its center, surrounded by a cloud of electrons. This model marked a significant departure from the previously held plum pudding model proposed by J.J. Thomson.
Rutherford’s model not only clarified the distribution of charge within the atom but also paved the way for future advancements in nuclear physics and quantum mechanics. His groundbreaking work set the stage for subsequent discoveries, including the identification of the neutron by James Chadwick in 1932.
Ernest Rutherford’s contributions to atomic physics and the discovery of the proton solidified his status as one of the most influential scientists of the 20th century. His experimental prowess, coupled with his profound insights, transformed our understanding of atomic structure and laid the foundation for further exploration of the subatomic world.
Rutherford’s proton discovery opened up new avenues of research, leading to advancements in nuclear physics, particle physics, and the development of atomic energy. His contributions earned him numerous accolades, including the Nobel Prize in Chemistry in 1908, and his scientific legacy continues to inspire generations of physicists worldwide.
Goldstein Discovered Proton:

In the realm of particle physics, there are numerous discoveries that have shaped our understanding of the fundamental building blocks of matter. One such monumental discovery is the identification of the proton, which played a pivotal role in unraveling the mysteries of atomic structure. This momentous breakthrough is credited to the brilliant scientist Eugen Goldstein, whose groundbreaking experiments in the late 19th century changed the course of scientific history.
Born on September 5, 1850, in Gleiwitz, Prussia (now Gliwice, Poland), Goldstein displayed an early aptitude for scientific pursuits. He studied physics and chemistry at the University of Breslau (now Wrocław University) and later pursued a career in academia. Goldstein’s insatiable curiosity and dedication to his craft led him to embark on a journey of discovery that would alter our understanding of atomic structure forever.
During the late 1800s, prevailing scientific theories held that atoms were indivisible, and the concept of subatomic particles was yet to be established. Goldstein’s groundbreaking experiments challenged these long-held beliefs and set the stage for a new era of understanding. In 1886, he developed a groundbreaking device known as the cathode-ray tube to investigate the properties of electric discharge in low-pressure gases.
Goldstein’s experiments with cathode rays led him to discover a new phenomenon that defied conventional wisdom. He noticed that when a high voltage was applied to the cathode-ray tube, a new type of ray was emitted in the opposite direction. These rays, which traveled in the opposite direction of the negatively charged cathode rays, came to be known as positive rays.
Further investigation revealed that these positive rays possessed unique properties that set them apart from their negative counterparts. Goldstein observed that these rays were deflected in the presence of electric and magnetic fields, indicating that they carried a positive charge. This groundbreaking discovery laid the foundation for the identification of a new fundamental particle, which Goldstein named the proton.
Goldstein’s discovery of the proton was a groundbreaking achievement in the field of particle physics. It provided compelling evidence for the existence of subatomic particles and shattered the prevailing belief in the indivisibility of atoms. His work not only revolutionized our understanding of atomic structure but also paved the way for subsequent discoveries, including the identification of the neutron and the development of the nuclear model of the atom.
The significance of Goldstein’s discovery cannot be overstated. It opened up new avenues for scientific exploration and propelled the field of particle physics into uncharted territories. His pioneering work with cathode rays and positive rays laid the groundwork for future researchers to delve deeper into the mysteries of the atomic nucleus and paved the way for subsequent breakthroughs in quantum mechanics and nuclear physics.
Goldstein’s contributions to science were not limited to the discovery of the proton. Throughout his career, he made significant contributions to the study of spectroscopy and gas discharges, and his work influenced a generation of scientists. His dedication to scientific inquiry and his unwavering pursuit of knowledge left an indelible mark on the field of physics.
In recognition of his groundbreaking achievements, Goldstein received numerous accolades and honors during his lifetime. He was awarded the Lieben Prize in 1897 by the Vienna Academy of Sciences and was elected a member of the Royal Swedish Academy of Sciences in 1902. Goldstein’s discoveries continue to inspire and shape the field of particle physics, and his legacy lives on in the minds of scientists around the world.
James Chadwick and His Proton Discovery:

Sir James Chadwick, a renowned British physicist, made significant contributions to our understanding of atomic structure and subatomic particles. Among his groundbreaking achievements was the discovery of the neutron, a neutral subatomic particle residing alongside the proton within the atomic nucleus. In this extensive article, we delve into Chadwick’s life, his pioneering experiments, and the profound implications of his neutron discovery. Join us on a captivating journey as we explore the scientific legacy of this remarkable scientist.
James Chadwick was born on October 20, 1891, in Bollington, England. He pursued his education at the University of Manchester, where he worked under the guidance of renowned physicist Ernest Rutherford. Chadwick’s talent for experimental physics and his meticulous approach to research quickly became evident during his academic years.
The Discovery of the Neutron
Chadwick’s most notable achievement came in 1932 when he made the groundbreaking discovery of the neutron. Building upon the knowledge of the positively charged proton and the negatively charged electron, Chadwick sought to uncover the missing piece of the atomic puzzle.
Through a series of elegant experiments involving the bombardment of beryllium with alpha particles, Chadwick observed an uncharged particle with a mass similar to that of the proton. This particle, which he named the neutron, provided the missing link in atomic theory and completed the understanding of atomic structure.
Chadwick’s neutron discovery had profound implications for the field of nuclear physics. It provided a new understanding of the stability and structure of atomic nuclei. The presence of neutrons, along with protons, was found to be crucial in preventing the repulsive forces between protons from destabilizing the atomic nucleus.
Furthermore, the neutron’s lack of charge meant it could penetrate atomic nuclei more effectively than charged particles, making it essential in studies of nuclear reactions and the fission process. Chadwick’s work paved the way for advancements in nuclear energy, atomic weapons, and the development of new scientific techniques, such as neutron scattering and radiography.
James Chadwick’s neutron discovery earned him numerous accolades and honors. In 1935, he was awarded the Hughes Medal by the Royal Society for his groundbreaking research. Later, in 1938, he received the Nobel Prize in Physics in recognition of his fundamental contributions to the field.
Chadwick’s neutron discovery not only solidified his status as a pioneering physicist but also provided a foundation for future research in atomic and nuclear physics. His work, combined with that of other influential scientists such as Rutherford and Chadwick’s mentor, Hans Geiger, laid the groundwork for the development of quantum mechanics and the understanding of fundamental particles and forces.
Chadwick’s later contributions to the Manhattan Project, the World War II research initiative that led to the development of the atomic bomb, further demonstrated the far-reaching impact of his discoveries.
J.J. Thomson and His Work on Protons:

Sir Joseph John Thomson, better known as J.J. Thomson, was a British physicist whose groundbreaking research revolutionized our understanding of atomic structure. Among his notable contributions was his work on protons, the positively charged subatomic particles found within atomic nuclei. In this comprehensive article, we delve into Thomson’s life, his pioneering experiments, and the profound impact of his discoveries. Join us on a captivating journey through the scientific achievements of this remarkable physicist.
J.J. Thomson was born on December 18, 1856, in Cheetham Hill, Manchester, England. He attended the Owens College (now the University of Manchester) and later pursued his doctoral studies at Trinity College, Cambridge. Under the guidance of his mentor, physicist George Gabriel Stokes, Thomson developed a keen interest in the properties of electricity and the nature of matter.
In the late 19th century, Thomson made a groundbreaking discovery that would forever change our understanding of atomic structure. Through his experiments with cathode rays, he demonstrated the existence of a negatively charged subatomic particle, which he named the electron.
Thomson’s experiments involved the use of cathode ray tubes and carefully designed apparatus to investigate the behavior of the mysterious rays. Through his observations of the deflection and bending of cathode rays in electric and magnetic fields, Thomson concluded that they were composed of particles carrying a negative charge, later identified as electrons.
Thomson’s Atomic Model: The Plum Pudding Model
Thomson’s discovery of the electron led to the development of a new atomic model known as the Plum Pudding Model or the Thomson Model. According to this model, atoms were envisioned as a positively charged “pudding” with embedded electrons distributed throughout, much like raisins in a plum pudding.
While Thomson’s work primarily focused on electrons, his investigations into atomic structure paved the way for future discoveries related to protons. By studying the behavior of particles within electric and magnetic fields, Thomson observed that atoms contained positive charge to counterbalance the negative charge of the electrons. This observation suggested the existence of a positively charged subatomic particle within the atom.
Although Thomson did not directly identify the particle as the proton, his work laid the foundation for further investigations into atomic structure and the role of protons in maintaining atomic stability.
J.J. Thomson’s groundbreaking work on protons, along with his discovery of the electron, had a profound impact on the field of atomic physics. His research fundamentally reshaped our understanding of atomic structure and set the stage for further advancements in subatomic particle exploration.
Thomson’s work on protons and electrons laid the groundwork for future scientists, including Ernest Rutherford, James Chadwick, and others, to expand our knowledge of atomic nuclei and the intricate balance between positively charged protons and negatively charged electrons.
Thomson’s contributions earned him numerous accolades, including the Nobel Prize in Physics in 1906, making him the first physicist to be honored with this prestigious award. His pioneering work continues to inspire scientists and serves as the foundation for modern theories in particle physics.
Proton Properties:

The proton, a positively charged subatomic particle, is a fundamental building block of matter. Its properties and characteristics have been extensively studied by physicists throughout history, leading to a deeper understanding of atomic structure and the fundamental forces that govern the universe. In this comprehensive article, we explore the properties of protons, their interactions, and their role in the structure of atoms. Join us on a captivating journey through the subatomic world as we unravel the mysteries of proton properties.
The proton is one of the three primary subatomic particles, alongside electrons and neutrons, that make up the atoms of elements. It carries a positive electric charge of +1 elementary charge, denoted as e. This charge is equal in magnitude but opposite in sign to that of the negatively charged electron.
The proton has a mass of approximately 1.67 x 10^-27 kilograms. It is roughly 1,836 times more massive than an electron. The size of a proton is on the order of femtometers, with a radius estimated to be around 0.87 femtometers.
Protons are located within the atomic nucleus, the central region of an atom. They carry a positive charge, which is balanced by the negatively charged electrons that orbit the nucleus. The number of protons in an atomic nucleus determines the element’s identity and is known as the atomic number. For example, hydrogen, the lightest element, consists of a single proton in its nucleus.
The positive charge of protons makes them subject to electromagnetic interactions. Protons repel other protons due to the electrostatic force of like charges, while they attract electrons due to the opposite charges. These interactions play a crucial role in determining the behavior of matter on both microscopic and macroscopic scales.
Protons are essential for the stability of atomic nuclei. The positively charged protons in the nucleus experience a repulsive force due to their like charges. However, the strong nuclear force, one of the fundamental forces in nature, overcomes this repulsion and binds the protons and neutrons together, keeping the atomic nucleus intact.
Different isotopes of an element have varying numbers of neutrons in their nuclei while maintaining the same number of protons. Isotopes with the same proton count but different neutron counts exhibit slightly different physical properties. The variation in neutron count affects the stability, nuclear decay, and other nuclear properties of isotopes.
Protons possess an intrinsic property called spin, which is associated with their angular momentum. Spin gives rise to a magnetic moment, allowing protons to interact with magnetic fields. This property is utilized in various scientific techniques, such as nuclear magnetic resonance (NMR) and magnetic resonance imaging (MRI).
According to modern theories in particle physics, protons are composite particles made up of smaller constituents called quarks. Protons consist of three quarks, specifically two up quarks and one down quark, bound together by the strong nuclear force. The interaction between quarks and the exchange of gluons, the carriers of the strong force, give rise to the overall properties of the proton.
Key Dates:

1897: J.J. Thomson and the Electron
In 1897, British physicist J.J. Thomson conducted experiments with cathode rays, leading to the discovery of the electron, a negatively charged subatomic particle. Thomson’s observations of the deflection of cathode rays in electric and magnetic fields provided crucial evidence for the existence of electrons within atoms.
1911: Ernest Rutherford and the Nucleus
In 1911, New Zealand-born physicist Ernest Rutherford conducted the famous gold foil experiment. By bombarding a thin gold foil with alpha particles, Rutherford and his team observed unexpected deflections, leading to the revolutionary conclusion that atoms possessed a dense, positively charged core known as the nucleus. This experiment laid the groundwork for further investigations into the nature of the nucleus and its constituents.
1919: Rutherford, Hans Geiger, and Ernest Marsden Probe the Atom
In 1919, Rutherford and his colleagues, Hans Geiger and Ernest Marsden, conducted experiments that shed light on the nature of atomic structure. By bombarding nitrogen gas with alpha particles, they observed unexpected deflections at larger angles than predicted. This discovery indicated the presence of a positively charged particle within the atom, later named the proton by Rutherford.
1920: The Term “Proton” Coined
In 1920, Ernest Rutherford officially coined the term “proton” to describe the newly discovered positively charged particle. Derived from the Greek word “protos” meaning “first” or “primary,” the term captured the essential role of the proton as a fundamental building block of matter.
1932: James Chadwick and the Neutron
In 1932, British physicist James Chadwick made a groundbreaking discovery that complemented our understanding of the proton. Through his experiments, Chadwick identified the neutron, a subatomic particle with no charge, residing alongside protons in the atomic nucleus. Chadwick’s discovery completed the picture of atomic nuclei and further enhanced our understanding of atomic structure.
Modern Era: Advancements in Particle Physics
Since the discovery of the proton, scientists have made significant advancements in understanding its properties and exploring the subatomic world. With the advent of particle accelerators, such as the Large Hadron Collider (LHC), researchers have studied proton collisions at high energies, unveiling new particles, interactions, and the fundamental laws of nature.
Conclusion
The invention or discovery of the proton, a fundamental subatomic particle, cannot be attributed to a single individual or event. Rather, the understanding of the proton evolved over time through the collective efforts of numerous scientists and their groundbreaking research. From the early work of J.J. Thomson, Ernest Rutherford, and James Chadwick to the advancements in particle physics and modern experiments, the journey of discovering the proton has been a collaborative endeavor.
J.J. Thomson’s discovery of the electron in 1897 provided the first indication of the existence of subatomic particles and their role in atomic structure. This paved the way for further investigations into the nature of the atom and its constituents. Ernest Rutherford’s gold foil experiment in 1911 revolutionized our understanding of the atomic nucleus, leading to the identification of the positively charged proton.
Rutherford’s experiments, coupled with the subsequent work of Hans Geiger and Ernest Marsden, provided crucial evidence for the presence of protons within atomic nuclei. Rutherford’s coinage of the term “proton” in 1920 solidified its recognition as a fundamental particle and highlighted its significance in the fabric of matter.
James Chadwick’s discovery of the neutron in 1932 complemented our understanding of the proton, completing the picture of atomic nuclei. Chadwick’s work expanded our knowledge of atomic structure and the delicate balance between protons and neutrons in maintaining nuclear stability.
It is important to note that the invention of the proton was not a singular event but rather a culmination of scientific advancements and discoveries. Countless researchers, physicists, and scientists have contributed to our understanding of the proton through their experiments, theories, and collaborations.
In conclusion, the invention of the proton is a testament to the collective efforts of the scientific community. The discoveries of the electron, the nucleus, and the neutron, along with the subsequent advancements in particle physics, have deepened our understanding of atomic structure and the fundamental building blocks of matter.
References:
- Thomson, J.J. “Cathode Rays.” Philosophical Magazine, 44(269), 293-316.
- Rutherford, E., Geiger, H., & Marsden, E. “Scattering of alpha Particles by Matter and the Structure of the Atom.” Philosophical Magazine, 21(125), 669-688.
- Rutherford, E. “The Proton.” Nature, 105(2639), 242-243.
- Chadwick, J. “Possible Existence of a Neutron.” Nature, 129(3252), 312.
- Chadwick, J. “The Neutron.” Proceedings of the Royal Society A, 136(830), 692-708.
- Einstein, A. “On the Electrodynamics of Moving Bodies.” Annalen der Physik, 17(10), 891-921.
- Bohr, N. “On the Constitution of Atoms and Molecules.” Philosophical Magazine, 26(151), 1-24.
- Fermi, E. “Versuch einer Theorie der β-Strahlen.” Zeitschrift für Physik, 88(3-4), 161-177.
- Wilson, C.T.R. “Cloud Chambers and the Tracks of Ionizing Particles.” Philosophical Transactions of the Royal Society A, 212(571-581), 277-295.
- Cockcroft, J.D., & Walton, E.T.S. “Experiments with High Velocity Positive Ions: II. The Disintegration of Elements by High Velocity Protons.” Proceedings of the Royal Society A, 136(830), 619-630.