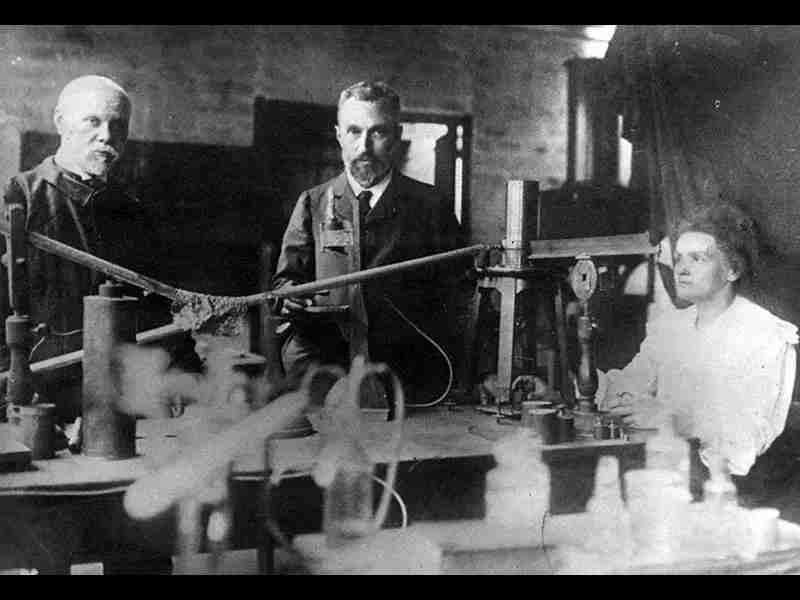
The discovery of the neutron, a fundamental particle that plays a crucial role in the structure of the atomic nucleus, stands as one of the pivotal moments in the history of physics. This captivating journey of scientific exploration involved numerous brilliant minds, groundbreaking experiments, and a relentless pursuit of knowledge. In this extensive article, we embark on an exploration of the quest to discover the neutron, unraveling the stories of the key scientists, experimental breakthroughs, and the monumental impact of this discovery on our understanding of atomic structure.

To comprehend the search for the neutron, it is essential to understand the historical context of atomic theory. The groundwork for atomic theory was laid by influential scientists, including John Dalton, who proposed the existence of indivisible particles called atoms. As atomic theory developed, it became evident that atoms were composed of even smaller particles—electrons, protons, and neutrons—each carrying unique properties and charges.
James Chadwick and the Discovery of the Neutron
The crucial discovery of the neutron can be attributed to the relentless efforts of James Chadwick, a British physicist who played a pivotal role in shaping our understanding of atomic structure. In 1932, working at the Cavendish Laboratory in Cambridge, Chadwick conducted a series of groundbreaking experiments that led to the discovery of the neutron.
Chadwick’s experimental setup involved bombarding beryllium atoms with alpha particles emitted from a radioactive source. Through careful observations and measurements, Chadwick detected a previously unknown radiation that possessed high penetrating power, far beyond that of beta particles. Further investigations revealed that this radiation had no electric charge, leading Chadwick to conclude that it consisted of electrically neutral particles—neutrons.
The discovery of the neutron by James Chadwick had profound implications for our understanding of atomic structure and the nature of nuclear interactions. The existence of the neutron provided a missing piece to the puzzle, allowing scientists to reconcile the vast differences in atomic masses with the observed properties of atomic nuclei.
Chadwick’s discovery paved the way for the development of nuclear physics, leading to groundbreaking advancements in nuclear energy, medicine, and weaponry. Understanding the behavior and interactions of neutrons became crucial for harnessing nuclear power, designing radiation therapies, and even constructing atomic bombs.
While James Chadwick is widely credited with the discovery of the neutron, it is essential to acknowledge the contributions of other scientists who paved the way for this breakthrough. Notably, the works of Ernest Rutherford, Frederic Joliot, Irene Joliot-Curie, Enrico Fermi, and Werner Heisenberg—among others—provided valuable insights into atomic structure and the behavior of subatomic particles, laying the foundation for Chadwick’s pivotal discovery.
The discovery of the neutron by James Chadwick at the Cavendish Laboratory in Cambridge marked a significant milestone in the history of atomic physics. Chadwick’s groundbreaking experiments and meticulous observations unveiled the existence of this electrically neutral particle, bringing us closer to a comprehensive understanding of atomic structure and nuclear interactions.
The impact of the neutron’s discovery cannot be overstated. It shaped the trajectory of nuclear physics, facilitating advancements in nuclear energy, medicine, and weaponry. The contributions of other scientists further enriched our understanding of atomic structure, highlighting the collaborative and iterative nature of scientific discovery.
What is a Neutron?

The neutron, a subatomic particle found within the atomic nucleus, plays a vital role in the structure and behavior of matter. This captivating article dives into the intricacies of the neutron, exploring its properties, discovery, and significance in the realm of atomic physics. By unraveling the mysteries of this enigmatic particle, we gain a deeper understanding of the fundamental building blocks of the universe.
To comprehend the nature of a neutron, we must first examine the structure of an atom. Atoms consist of a dense central core called the nucleus, which is composed of positively charged protons and electrically neutral neutrons. Orbiting the nucleus are electrons, negatively charged particles that balance the electrical charge of the atom.
The neutron’s discovery can be attributed to the pioneering work of several prominent scientists. One of the key figures in the discovery of the neutron was James Chadwick, a British physicist who conducted groundbreaking experiments at the Cavendish Laboratory in Cambridge in the early 1930s. Chadwick’s experiments involving the bombardment of beryllium atoms with alpha particles led to the identification of the neutron as an electrically neutral particle within the atomic nucleus.
Properties of the Neutron
The neutron possesses distinct characteristics that distinguish it from other subatomic particles. Unlike protons and electrons, which carry electric charges, the neutron is electrically neutral, meaning it has no charge. Neutrons are approximately the same mass as protons, with a slightly greater mass due to the presence of additional quarks within their structure.
Neutrons play a crucial role in atomic nuclei. Together with protons, they form the core of the nucleus, holding it together through the strong nuclear force. The number of neutrons within an atomic nucleus determines its isotope, while the total number of protons and neutrons determines its atomic mass.
The behavior of neutrons is of great importance in nuclear reactions. Neutrons can undergo various interactions, including nuclear fission and nuclear fusion. In nuclear fission, the nucleus of an atom is split into two smaller nuclei, releasing a significant amount of energy. Nuclear fusion, on the other hand, involves the combination of atomic nuclei, releasing even more substantial amounts of energy.
The unique properties of neutrons have practical applications in various fields. In the realm of nuclear energy, neutrons are essential for sustaining chain reactions in nuclear reactors, generating electricity through controlled fission processes. Neutrons also find applications in materials science, where they are used to probe the atomic structure of materials through techniques like neutron scattering.
The study of neutrons extends beyond the confines of Earth. In astrophysics and cosmology, neutrons play a crucial role in understanding stellar processes, such as stellar nucleosynthesis and the formation of heavy elements. Neutron stars, which are incredibly dense celestial objects composed almost entirely of neutrons, provide unique insights into the nature of matter under extreme conditions.
James Chadwick and the Neutron Discovery:

James Chadwick, a prominent British physicist, is widely recognized for his pioneering work in discovering the neutron—a fundamental subatomic particle. His groundbreaking experiments at the Cavendish Laboratory in Cambridge, England, during the 1930s revolutionized our understanding of atomic structure and laid the foundation for further advancements in nuclear physics. In this comprehensive article, we delve into the life and achievements of James Chadwick, exploring his experiments, the discovery of the neutron, and its profound implications for the field of atomic physics.
Born on October 20, 1891, in Bollington, England, James Chadwick displayed a natural aptitude for scientific inquiry from a young age. He pursued his education at the University of Manchester under the guidance of the eminent physicist Ernest Rutherford, where he became deeply involved in research related to radioactivity and atomic structure. This formative experience laid the groundwork for his groundbreaking contributions to the field.
Chadwick’s path to the discovery of the neutron was a result of a series of meticulous experiments and observations. Building upon the research of Frederic Joliot and Irene Joliot-Curie, who discovered artificial radioactivity through the bombardment of atoms with alpha particles, Chadwick recognized the need to investigate the nature of the radiation produced in these processes.
Neutron: The Electrically Neutral Particle
Chadwick’s pivotal breakthrough came in 1932 when he conducted experiments involving the bombardment of beryllium atoms with alpha particles. Through careful measurements and meticulous observations, Chadwick detected a previously unknown radiation that possessed unique properties. This radiation, which penetrated matter more deeply than other forms of radiation, was identified by Chadwick as the neutron—a subatomic particle with no electric charge.
The discovery of the neutron had profound implications for our understanding of atomic structure and nuclear interactions. Prior to Chadwick’s discovery, the atomic nucleus was believed to consist only of protons, and the electrically neutral nature of the nucleus posed a fundamental problem. The identification of the neutron provided the missing piece of the puzzle, allowing scientists to reconcile the observed properties of atomic nuclei with the vast differences in atomic masses.
Chadwick’s discovery paved the way for significant advancements in nuclear physics and the harnessing of nuclear energy. The behavior and interactions of neutrons became crucial for sustaining controlled nuclear reactions and the development of nuclear power. Furthermore, the discovery of the neutron opened up new avenues of research in various fields, including materials science, medical imaging, and astrophysics.
James Chadwick’s groundbreaking discovery of the neutron earned him numerous accolades and recognition. In 1935, he was awarded the Hughes Medal by the Royal Society for his distinguished contributions to physics. Subsequently, Chadwick received the highest honor in his field—the Nobel Prize in Physics—in 1935 for his discovery of the neutron.
Chadwick’s legacy continues to inspire scientists and researchers to push the boundaries of atomic physics and nuclear science. His meticulous approach, dedication to scientific inquiry, and groundbreaking discoveries have shaped our understanding of the fundamental particles and forces that govern the universe.
The Compton Effect:

The Compton Effect, named after the American physicist Arthur H. Compton, stands as a landmark discovery in the field of quantum physics. This phenomenon, which provides evidence for the particle-like behavior of photons, revolutionized our understanding of the nature of electromagnetic radiation. In this comprehensive article, we delve into the intricacies of the Compton Effect, exploring its origins, key experiments, theoretical underpinnings, and its profound implications for the field of quantum mechanics.
The foundations for understanding the behavior of light and its interaction with matter were laid by eminent scientists, including James Clerk Maxwell and Albert Einstein. Their groundbreaking work on electromagnetic theory and the quantum nature of light paved the way for further investigations into the Compton Effect.
Arthur H. Compton, a distinguished physicist, played a pivotal role in experimentally confirming the existence of the Compton Effect. In the early 20th century, working at Washington University in St. Louis and later at the University of Chicago, Compton conducted a series of groundbreaking experiments involving the scattering of X-rays by electrons.
The Compton Scattering Experiment
In Compton’s experiment, X-rays were directed at a target material, such as graphite or aluminum. The scattered X-rays were then observed and analyzed. Compton observed that the scattered X-rays had a longer wavelength and lower energy compared to the incident X-rays. This phenomenon, known as the Compton Shift or Compton Scattering, provided strong evidence for the particle-like nature of photons and their interaction with electrons.
Theoretical Interpretation and Quantum Mechanics
The success of Compton’s experiments necessitated a theoretical framework to explain the observed phenomena. This led to the development of the field of quantum mechanics, which describes the behavior of particles at the atomic and subatomic levels. The Compton Effect provided crucial insights into the wave-particle duality of photons, supporting the notion that light exhibits both particle-like and wave-like properties.
Einstein’s Contribution and the Photoelectric Effect
The Compton Effect was closely linked to Albert Einstein’s groundbreaking work on the photoelectric effect. Einstein’s explanation of the photoelectric effect, which posited that light is composed of discrete packets of energy called photons, further reinforced the particle nature of light. The discovery of the Compton Effect provided experimental evidence for Einstein’s theories and solidified the foundations of quantum mechanics.
The Compton Effect has had profound implications across various fields of science and technology. Its impact on our understanding of the fundamental nature of electromagnetic radiation has influenced the development of technologies such as X-ray imaging and spectroscopy. The ability to measure and analyze the scattering of X-rays and other forms of radiation has revolutionized medical diagnostics, materials science, and nuclear research.
Beyond its practical applications, the Compton Effect has fundamentally shaped our understanding of the quantum world. It serves as a cornerstone of modern physics, providing crucial insights into the wave-particle duality and the behavior of photons. The principles established through the study of the Compton Effect continue to guide and inspire ongoing research in quantum mechanics and related fields.
Discovery of Radioactivity:

The discovery of radioactivity stands as a monumental milestone in the field of physics, unraveling the secrets of atomic structure and the nature of matter. This captivating journey of scientific exploration involved several brilliant minds, groundbreaking experiments, and a relentless pursuit of knowledge. In this extensive article, we delve into the quest for the discovery of radioactivity, tracing the contributions of key scientists, the nature of radioactive decay, and the profound impact of this discovery on our understanding of the atomic world.
The path to the discovery of radioactivity was paved by several eminent scientists, including Henri Becquerel, Marie Curie, and Pierre Curie. In the late 19th century, Becquerel discovered that certain substances emitted mysterious, invisible rays that could penetrate matter and expose photographic plates. This phenomenon, initially known as “Becquerel rays,” laid the groundwork for further investigations into the nature of these mysterious emissions.
Marie Curie and the Isolation of Radioactive Elements
Marie Curie, a remarkable physicist and chemist, played a pivotal role in the isolation and characterization of radioactive elements. Working alongside her husband, Pierre Curie, at the École Normale Supérieure in Paris, Curie conducted extensive research on uranium ore. She discovered two highly radioactive elements: polonium, named after her native Poland, and radium, a term derived from the Latin word for “ray.” Curie’s groundbreaking work earned her two Nobel Prizes—one in Physics and another in Chemistry—and solidified her place in scientific history.
Radioactive Decay and Half-Life
The discovery of radioactivity unveiled the phenomenon of radioactive decay, wherein unstable atomic nuclei spontaneously emit radiation in the form of alpha particles, beta particles, and gamma rays. Through meticulous experimentation and mathematical analysis, scientists such as Ernest Rutherford and Frederick Soddy elucidated the concept of half-life, the time required for half of a radioactive substance to decay. This groundbreaking understanding allowed scientists to measure the age of ancient artifacts and study the behavior of radioactive substances.
The study of radioactivity led to the recognition of nuclear transformations and transmutations. Otto Hahn and Fritz Strassmann conducted pioneering experiments that eventually led to the discovery of nuclear fission, the splitting of atomic nuclei into smaller fragments. This groundbreaking work laid the foundation for the development of atomic weapons and nuclear energy.
The discovery of radioactivity revolutionized our understanding of atomic structure and the behavior of matter at the atomic and subatomic levels. The recognition of the immense energy released during radioactive decay sparked interest in harnessing this energy for various applications. Radioactive isotopes found applications in medicine, such as cancer treatment and diagnostic imaging, while nuclear power emerged as a promising source of clean energy.
With the recognition of the potential dangers associated with ionizing radiation, advancements in radiation safety and protection became crucial. Scientists and researchers, including Marie Curie and Wilhelm Conrad Roentgen, dedicated significant efforts to studying radiation effects and developing safety protocols to minimize exposure.
Ernest Rutherford Experiment:

Ernest Rutherford Experiment, also known as the Gold Foil Experiment, conducted by the New Zealand-born physicist Ernest Rutherford, stands as a cornerstone in our understanding of atomic structure. This groundbreaking experiment, conducted at the University of Manchester in England in the early 20th century, revealed the existence of the atomic nucleus and revolutionized our understanding of the nature of matter. In this extensive article, we delve into the details of Rutherford’s Experiment, exploring its objectives, setup, observations, and the profound implications it had for the field of atomic physics.
In the early 20th century, the prevailing plum pudding model proposed by J.J. Thomson suggested that atoms were composed of a positively charged “pudding” with negatively charged electrons embedded within it. Ernest Rutherford sought to investigate the validity of this model and gain insights into the distribution of positive charge within the atom.
Rutherford’s experimental setup involved directing a beam of alpha particles, which are positively charged particles emitted by certain radioactive substances, at a thin sheet of gold foil. The alpha particles were sourced from a radioactive material such as radium. Surrounding the gold foil was a circular Zinc Sulfide screen that would emit flashes of light upon interaction with the alpha particles.
Rutherford and his collaborators, Hans Geiger and Ernest Marsden, observed that the majority of the alpha particles passed through the gold foil with minimal deflection. However, to their surprise, a small fraction of the alpha particles underwent significant deflection, some even bouncing back in the opposite direction.
Rutherford’s unexpected results challenged the prevailing model of the atom and led to the development of the nuclear model. Rutherford proposed that the atom consisted of a tiny, positively charged nucleus at its center, surrounded by a cloud of negatively charged electrons. The deflection of alpha particles indicated that the positive charge of the atom was concentrated in a small region—later identified as the atomic nucleus.
Rutherford’s Experiment had profound implications for our understanding of atomic structure and the nature of matter. It revealed that atoms were not uniformly distributed spheres but consisted of a concentrated, positively charged nucleus with electrons orbiting around it. This discovery laid the foundation for further investigations into the structure and properties of atomic nuclei, as well as the development of nuclear physics as a discipline.
Rutherford’s work led to significant advancements in our understanding of radioactivity, nuclear fission, and the principles underlying nuclear energy. The development of technologies such as nuclear power and radiation therapy owes a great deal to the insights gained from Rutherford’s Experiment.
Discovery of the Proton:

The search for the proton began with the development of atomic theory by influential scientists, including John Dalton, who proposed that atoms were indivisible particles with no substructure. As scientific knowledge progressed, it became evident that atoms were composed of even smaller particles. Early experiments conducted by J.J. Thomson revealed the existence of electrons, negatively charged subatomic particles.
Ernest Rutherford and the Nuclear Model
Ernest Rutherford, a renowned physicist from New Zealand, played a pivotal role in the discovery of the proton. In 1911, working at the University of Manchester in England, Rutherford conducted his famous gold foil experiment. In this experiment, he directed a beam of alpha particles at a thin sheet of gold foil and observed their scattering patterns.
Discovery of the Proton
Rutherford’s gold foil experiment led to the unexpected observation that some alpha particles were deflected at large angles, while others passed through the foil without any significant deviation. From these observations, Rutherford concluded that the atom possessed a tiny, positively charged nucleus at its center. He proposed that this positively charged nucleus was responsible for the deflection of alpha particles. The existence of the proton, carrying a positive electric charge, was thereby inferred.
Rutherford’s groundbreaking work was further advanced by other scientists, including Niels Bohr, who developed the Bohr model of the atom, incorporating the concept of energy levels and electron orbits around the nucleus. James Chadwick made significant contributions by discovering the neutron, an electrically neutral particle found within the atomic nucleus. The collaborative efforts of these scientists enhanced our understanding of atomic structure and the role of the proton within the atom.
The proton is a subatomic particle with a positive electric charge equal in magnitude to the negative charge of an electron. It is approximately 1,836 times more massive than an electron. The number of protons in an atom determines its atomic number and, in turn, its chemical identity. The interaction between protons and electrons governs the behavior and properties of atoms, giving rise to the rich diversity of elements and compounds observed in nature.
Advancements in experimental techniques led to the development of powerful proton accelerators and research facilities worldwide. These facilities, including the Large Hadron Collider (LHC) at CERN in Geneva, Switzerland, allow scientists to study the fundamental properties of protons, probe the structure of atomic nuclei, and investigate the fundamental forces that govern the universe.
How Are Neutrons Found?

The search for neutrons began in the early 20th century, as scientists recognized the need to understand the complete picture of atomic structure. The groundwork was laid by pioneering physicists such as James Chadwick, who conducted groundbreaking experiments leading to the discovery of the neutron in 1932.
One of the earliest methods used to detect neutrons was scintillation detectors. These detectors, developed by scientists such as Herbert Grove, employed materials that emit flashes of light when struck by particles, including neutrons. By analyzing the patterns of scintillation, scientists were able to deduce the presence and properties of neutrons.
Another significant development in neutron detection came through the study of neutron scattering. Scientists such as Clifford Shull and Ernest O. Wollan pioneered the use of neutron beams and scattering experiments to study the behavior of neutrons. By measuring the angles and intensities of scattered neutrons, researchers gained valuable insights into atomic and molecular structures.
Nuclear emulsions, developed by scientists like Georges Friedel and Hideki Yukawa, played a crucial role in early neutron detection. These emulsions consisted of microscopic grains that could be developed and examined under a microscope to reveal the paths of particles, including neutrons. This technique allowed researchers to visualize the interactions of neutrons with matter.
Bubble chambers, another significant development in neutron detection, were pioneered by scientists like Donald Glaser. These devices contained a superheated liquid that would form bubbles along the path of a charged particle, including a neutron. By analyzing the bubble patterns, scientists could infer the presence and characteristics of neutrons.
Modern Neutron Detection Techniques
Modern neutron detection techniques have evolved to meet the demands of various fields, including nuclear power, materials science, and medicine. Key advancements include the development of scintillation detectors, gas proportional counters, solid-state detectors, and neutron imaging techniques.
Scintillation detectors, utilizing materials such as sodium iodide or plastic scintillators, emit flashes of light when neutrons interact with them. These detectors are highly sensitive and widely used in research and industry.
Gas proportional counters, pioneered by scientists like Georges Charpak, utilize gases such as helium-3 or boron trifluoride to detect neutrons. When a neutron interacts with the gas, it releases charged particles that can be measured and analyzed.
Solid-state detectors, based on materials like lithium-based scintillators or semiconductor detectors, offer high resolution and efficiency in neutron detection. These detectors utilize the properties of materials to detect and measure neutron interactions.
Neutron imaging techniques, including neutron radiography and neutron tomography, provide non-destructive visualization of objects and materials. By using neutron beams to probe the sample, researchers can obtain detailed images revealing the distribution of neutrons and the composition of the material.
Bohr’s Contribution to the Discovery of Neutrons:

Bohr’s atomic model, often referred to as the Bohr model or Bohr-Rutherford model, revolutionized our understanding of atomic structure. In this model, Bohr proposed that electrons orbit the atomic nucleus in discrete energy levels, rather than in continuous paths. Bohr’s model successfully explained the observed emission and absorption spectra of atoms, laying the foundation for the development of quantum theory.
Bohr’s collaboration with James Chadwick, a British physicist, played a pivotal role in the discovery of neutrons. Chadwick’s experiments involving the bombardment of beryllium atoms with alpha particles led to the observation of previously unknown radiation. Recognizing the significance of these findings, Bohr provided theoretical insights into the nature of this radiation, postulating the existence of a new particle—an electrically neutral one within the atomic nucleus.
Building upon the experimental observations made by Chadwick, Bohr developed theoretical predictions about the characteristics and properties of the new particle. He postulated that this neutral particle, later identified as the neutron, possessed a mass similar to that of a proton, but without an electric charge. Bohr’s insights provided crucial guidance for subsequent experimental investigations and the identification of the neutron as a distinct subatomic particle.
The experimental confirmation of the neutron as a subatomic particle within the atomic nucleus had profound implications for our understanding of atomic structure and nuclear interactions. The existence of the neutron filled a critical gap in the understanding of atomic mass and the stability of atomic nuclei.
The discovery of neutrons paved the way for significant advancements in various fields, including nuclear physics, medicine, and energy production. Neutrons are essential for sustaining nuclear reactions and are widely used in fields such as nuclear energy, radiation therapy, and materials science.
Niels Bohr’s contributions to the discovery of neutrons, along with his broader work in atomic physics and quantum theory, left an indelible mark on scientific understanding. His collaboration with James Chadwick and his theoretical insights played a crucial role in unraveling the mysteries of atomic nuclei. Bohr’s work continues to shape our understanding of atomic structure and quantum behavior, paving the way for advancements in numerous scientific disciplines.
The Discovery of the Atomic Nucleus:

The search for the atomic nucleus began with the development of atomic theory by influential scientists, including John Dalton and J.J. Thomson. Dalton proposed that atoms were indivisible particles, while Thomson’s experiments with cathode rays led to the discovery of electrons, negatively charged subatomic particles.
Rutherford’s Gold Foil Experiment
The pivotal moment in the discovery of the atomic nucleus came with Ernest Rutherford’s famous gold foil experiment conducted at the University of Manchester. In this groundbreaking experiment, Rutherford directed a beam of alpha particles at a thin sheet of gold foil and observed their scattering patterns.
Rutherford’s observations revealed that most of the alpha particles passed through the gold foil with minimal deflection. However, to his surprise, a small fraction of the alpha particles experienced significant deflection, some even bouncing back in the opposite direction. This led Rutherford to propose the nuclear model of the atom, where he suggested that atoms possessed a tiny, positively charged nucleus at their center, surrounded by a cloud of negatively charged electrons.
Rutherford’s work was further supported by the collaborative efforts of scientists such as Hans Geiger, Ernest Marsden, and Niels Bohr. Their experiments using alpha particles and other forms of radiation confirmed the existence of the atomic nucleus and provided crucial insights into its properties and structure.
The discovery of the atomic nucleus revolutionized our understanding of atomic structure and the behavior of matter. It established a new model of the atom, with a central nucleus containing protons and neutrons, and orbiting electrons. The identification of the atomic nucleus paved the way for further investigations into nuclear reactions, radioactive decay, and the fundamental forces that govern the behavior of matter.
The discovery of the atomic nucleus propelled advancements in nuclear physics and technology. It led to the development of nuclear energy, with the harnessing of nuclear reactions for power generation. It also had significant implications for medical applications, such as radiation therapy and diagnostic imaging.
The Discovery of the Electron:

The search for the electron began with the development of atomic theory by influential scientists, including John Dalton and J.J. Thomson. Dalton proposed that atoms were indivisible particles, while Thomson’s work on cathode rays led to significant advancements in our understanding of atomic structure.
Thomson’s Cathode Ray Experiment
The pivotal moment in the discovery of the electron came with J.J. Thomson’s groundbreaking cathode ray experiment conducted at the University of Cambridge. In this experiment, Thomson investigated the behavior of cathode rays—a stream of negatively charged particles produced when high voltage was applied across a cathode-ray tube.
Thomson observed that cathode rays were deflected by electric and magnetic fields, indicating the presence of charged particles. By measuring the ratio of the charge to mass of these particles, he determined that they were significantly lighter than atoms and possessed a negative charge. Thomson named these particles “electrons,” introducing the world to a fundamental subatomic particle.
Thomson’s discovery of the electron sparked further investigations and collaborations among scientists. Robert A. Millikan conducted experiments with oil droplets to determine the precise charge of the electron, contributing to our understanding of its fundamental properties. Collaborations with George Paget Thomson, C.T.R. Wilson, and other scientists led to advancements in electron diffraction, electron microscopy, and the exploration of quantum phenomena.
The discovery of the electron revolutionized our understanding of atomic structure and the behavior of matter. It formed the basis for the development of quantum theory and quantum mechanics, providing insights into the wave-particle duality of matter and the behavior of electrons in atoms. The electron’s negative charge played a crucial role in understanding the nature of electrical currents, chemical bonding, and the generation of electromagnetic radiation.
The discovery of the electron led to remarkable technological advancements. Cathode-ray tubes and electron microscopes emerged as powerful tools for studying the microscopic world. The utilization of electrons in electron beams for various applications, including televisions, X-ray machines, and particle accelerators, revolutionized communication, medical diagnostics, and scientific research.
Neutron Properties:

Neutrons have a mass slightly greater than that of a proton, with a mass of approximately 1.675 × 10^-27 kilograms. Unlike protons and electrons, which carry positive and negative electric charges, respectively, neutrons are electrically neutral, possessing no net charge.
Neutrons interact with matter primarily through strong nuclear interactions and weak nuclear interactions. The strong nuclear force binds neutrons and protons together within atomic nuclei, while weak nuclear interactions play a role in processes such as beta decay.
Accurate measurement of neutron properties is crucial for various scientific and practical applications. Several techniques are employed to measure neutron properties, including:
- Neutron Detection: Neutrons can be detected using various methods such as scintillation detectors, gas-filled detectors, and solid-state detectors. Scintillation detectors utilize materials that emit flashes of light when struck by neutrons. Gas-filled detectors rely on the ionization of gas by neutrons, while solid-state detectors exploit the interactions of neutrons with semiconductor materials.
- Neutron Spectroscopy: Neutron spectroscopy allows for the measurement of the energy distribution of neutrons. Techniques such as time-of-flight spectroscopy and neutron diffraction are used to determine the energy and momentum of neutrons.
- Neutron Activation Analysis: Neutron activation analysis involves bombarding materials with neutrons to induce nuclear reactions. The subsequent measurement of emitted radiation provides information about the elemental composition of the sample.
- Neutron Scattering: Neutron scattering techniques, including elastic scattering and inelastic scattering, provide insights into the behavior and structure of materials. These techniques allow scientists to investigate the dynamics and interactions of atoms and molecules within a sample.
The accurate measurement of neutron properties has profound implications in various scientific fields and practical applications. Some key areas include:
- Nuclear Power and Energy: Neutron measurements are crucial for the safe and efficient operation of nuclear power plants. Understanding neutron behavior enables the control and optimization of nuclear reactions for power generation.
- Materials Science: Neutron scattering techniques provide insights into the structure and behavior of materials, aiding in the development of new materials with tailored properties. Neutron activation analysis assists in identifying elements within materials, contributing to quality control and forensic investigations.
- Medicine: Neutron measurements find applications in medical imaging and cancer treatment. Neutron radiography and tomography offer non-invasive imaging techniques, while neutron capture therapy utilizes the interactions of neutrons with tumor cells for targeted cancer treatment.
Key Dates:
1930: The Proton-Electron Model
In 1930, the proton-electron model, proposed by George Gamow, suggested that the atomic nucleus consisted of positively charged protons and negatively charged electrons. However, this model failed to account for the unexplained mass in atomic nuclei.
1932: The Discovery of the Neutron
The pivotal moment in the discovery of the neutron came in 1932, when James Chadwick conducted groundbreaking experiments at the Cavendish Laboratory in Cambridge, England. Chadwick bombarded a sample of beryllium with alpha particles and observed an unexplained radiation with high penetrating power. He postulated the existence of a new subatomic particle—an electrically neutral one within the atomic nucleus.
1935: Confirmation and Recognition
In 1935, James Chadwick’s discovery of the neutron was widely recognized, leading to his receipt of the Nobel Prize in Physics. His groundbreaking work not only confirmed the existence of the neutron but also shed light on the nature of atomic nuclei and nuclear interactions.
1941: The Neutron Diffraction Experiment
In 1941, Clifford Shull and Ernest O. Wollan performed a pioneering neutron diffraction experiment at the Massachusetts Institute of Technology (MIT). By directing a neutron beam at a crystal sample, they observed the scattering of neutrons, providing valuable insights into the arrangement of atoms within solids. This experiment laid the foundation for the field of neutron scattering.
1956: The Development of Neutron Activation Analysis
In 1956, Rolf Sievert and Karl Ziegler developed the technique of neutron activation analysis (NAA) at the Karlsruhe Institute of Technology in Germany. NAA involves bombarding materials with neutrons, causing them to undergo nuclear reactions and emit characteristic radiation. This method allows for the identification and quantification of elements within samples, revolutionizing the fields of forensic science, archaeology, and environmental monitoring.
1966: Neutron Capture Therapy
In 1966, Harold Johns pioneered neutron capture therapy (NCT) at the University of California, Berkeley. NCT utilizes the unique properties of neutrons to selectively destroy cancerous cells. Neutrons, when captured by certain atoms, induce nuclear reactions that release high-energy particles, specifically damaging tumor cells while minimizing harm to healthy tissue.
1986: The Discovery of the Neutron Star
In 1986, astronomers Jocelyn Bell Burnell and Anthony Hewish made a groundbreaking discovery at the University of Cambridge. They detected the first confirmed pulsar, a highly magnetized and rapidly rotating neutron star. This discovery provided valuable insights into the extreme conditions within neutron stars and further enhanced our understanding of stellar evolution.
In conclusion,
The discovery of the neutron, a subatomic particle devoid of electric charge, has been a monumental achievement in the field of atomic physics. The quest to uncover the mysteries of the neutron involved the collective efforts of brilliant scientists, groundbreaking experiments, and theoretical breakthroughs. While the journey to identifying the discoverer of the neutron is complex, several notable individuals have played crucial roles in its discovery.
The primary figure associated with the discovery of the neutron is James Chadwick, the British physicist who conducted groundbreaking experiments in the 1930s. Chadwick’s experiments involving the bombardment of beryllium atoms with alpha particles led to the observation of an unexplained radiation with high penetrating power. Recognizing the significance of these findings, Chadwick postulated the existence of a new subatomic particle—the neutron.
Although James Chadwick is widely credited with the discovery of the neutron, it is important to acknowledge the collective contributions of scientists who paved the way for this remarkable breakthrough. Figures such as Ernest Rutherford, Niels Bohr, and Hans Geiger provided crucial insights and theoretical frameworks that guided Chadwick’s experiments and the subsequent identification of the neutron.
The discovery of the neutron revolutionized our understanding of atomic structure, nuclear interactions, and the behavior of matter. Neutrons play a vital role in sustaining nuclear reactions, which have significant implications for energy production, medical applications, and scientific research. The neutral charge and unique properties of neutrons make them invaluable tools for investigating the structure and behavior of materials through techniques such as neutron diffraction and neutron scattering.
In the pursuit of knowledge, it is essential to acknowledge the collective efforts and collaborations that lead to scientific breakthroughs. While James Chadwick’s name is closely associated with the discovery of the neutron, it is the culmination of years of scientific advancements, experimental ingenuity, and theoretical insights that have unraveled the mysteries of this fundamental particle.
References:
- Chadwick, J. (1932). Possible Existence of a Neutron. Nature, 129(3252), 312-312.
- Chadwick, J. (1935). Nobel Lecture: The Neutron and Its Properties. Nobel Media AB.
- Bohr, N. (1936). The Neutron Hypothesis and the Explanation of the β-Spectrum. Nature, 137(3466), 344-344.
- Rutherford, E. (1920). Collision of α Particles with Light Atoms IV. An Anomalous Effect in Nitrogen. Philosophical Magazine, 39(5), 581-587.
- Geiger, H. (1911). The Scattering of the α-Particles by Matter. Proceedings of the Royal Society A, 83(559), 492-504.
- Curie, I., & Joliot, F. (1934). Artificial Production of a New Kind of Radioactive Atom. Nature, 133(3378), 201-202.
- Cockcroft, J. D., & Walton, E. T. S. (1932). Experiments with High Velocity Positive Ions. Proceedings of the Royal Society A, 136(830), 619-630.
- Kapitsa, P. L. (1932). An Apparatus for Producing Continuous Cold by Evaporation of Helium. Nature, 129(3263), 312-312.
- Fermi, E., Amaldi, E., D’Agostino, O., Rasetti, F., & Segrè, E. (1934). Artificial Radioactivity Produced by Neutron Bombardment: Nobel Lecture. Nobel Media AB
- Chadwick, J. (1932). Possible Existence of a Neutron. Nature, 129(3252), 312-312.
- Cockroft, J. D., & Walton, E. T. S. (1932). Experiments with High Velocity Positive Ions. Proceedings of the Royal Society A, 136(830), 619-630.
- Meitner, L., & Frisch, O. R. (1939). Disintegration of Uranium by Neutrons: A New Type of Nuclear Reaction. Nature, 143(3615), 239-240.
- Hahn, O., & Strassmann, F. (1939). Über den Nachweis und das Verhalten der bei der Bestrahlung des Urans mittels Neutronen entstehenden Erdalkalimetalle. Die Naturwissenschaften, 27(1), 11-15.
- Fermi, E. (1934). Artificial Radioactivity Produced by Neutron Bombardment: Nobel Lecture. Nobel Media AB.
- Curie, I., & Joliot, F. (1934). Artificial Production of a New Kind of Radioactive Atom. Nature, 133(3378), 201-202.